Marco Pizzolato
Microscopic Propagator Imaging (MPI) with Diffusion MRI
Feb 28, 2025



Abstract:We propose Microscopic Propagator Imaging (MPI) as a novel method to retrieve the indices of the microscopic propagator which is the probability density function of water displacements due to diffusion within the nervous tissue microstructures. Unlike the Ensemble Average Propagator indices or the Diffusion Tensor Imaging metrics, MPI indices are independent from the mesoscopic organization of the tissue such as the presence of multiple axonal bundle directions and orientation dispersion. As a consequence, MPI indices are more specific to the volumes, sizes, and types of microstructures, like axons and cells, that are present in the tissue. Thus, changes in MPI indices can be more directly linked to alterations in the presence and integrity of microstructures themselves. The methodology behind MPI is rooted on zonal modeling of spherical harmonics, signal simulation, and machine learning regression, and is demonstrated on both synthetic and Human Diffusion MRI data.
Axial and radial axonal diffusivities from single encoding strongly diffusion-weighted MRI
Jul 06, 2022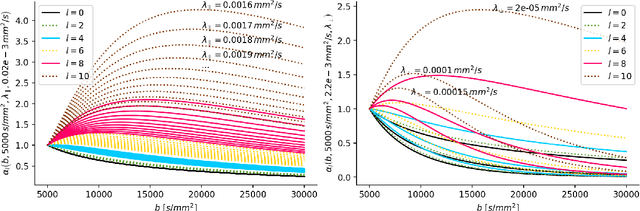
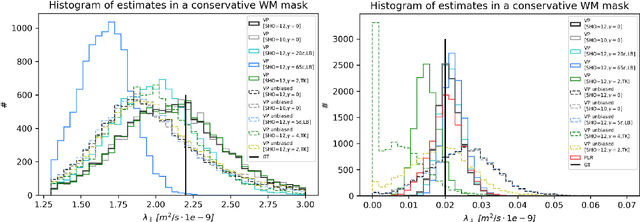
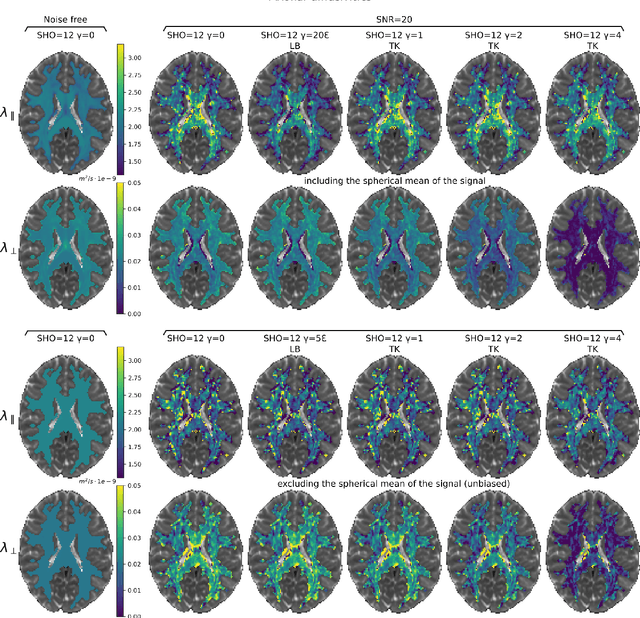
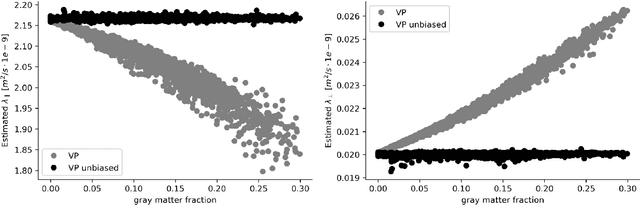
Abstract:We enable the estimation of the per-axon axial diffusivity from single encoding, strongly diffusion-weighted, pulsed gradient spin echo data. Additionally, we improve the estimation of the per-axon radial diffusivity compared to estimates based on spherical averaging. The use of strong diffusion weightings in magnetic resonance imaging (MRI) allows to approximate the signal in white matter as the sum of the contributions from axons. At the same time, spherical averaging leads to a major simplification of the modeling by removing the need to explicitly account for the unknown orientation distribution of axons. However, the spherically averaged signal acquired at strong diffusion weightings is not sensitive to the axial diffusivity, which cannot therefore be estimated. After revising existing theory, we introduce a new general method for the estimation of both axonal diffusivities at strong diffusion weightings based on zonal harmonics modeling. We additionally show how this could lead to estimates that are free from partial volume bias with, for instance, gray matter. We test the method on publicly available data from the MGH Adult Diffusion Human Connectome project dataset. We report reference values of axonal diffusivities based on 34 subjects, and derive estimates of axonal radii. We address the estimation problem also from the angle of the required data preprocessing, the presence of biases related to modeling assumptions, current limitations, and future possibilities.
Multi-compartment diffusion MRI, T2 relaxometry and myelin water imaging as neuroimaging descriptors for anomalous tissue detection
Apr 15, 2021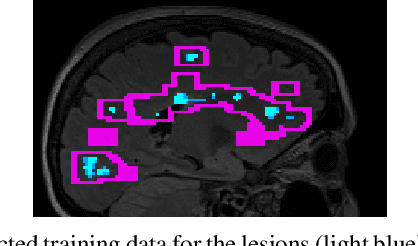
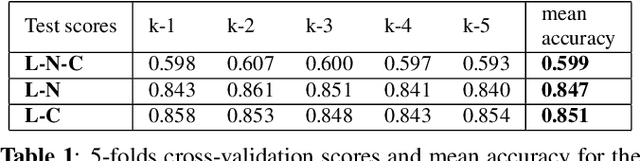
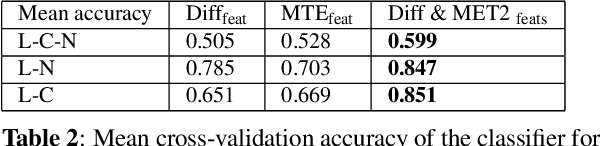
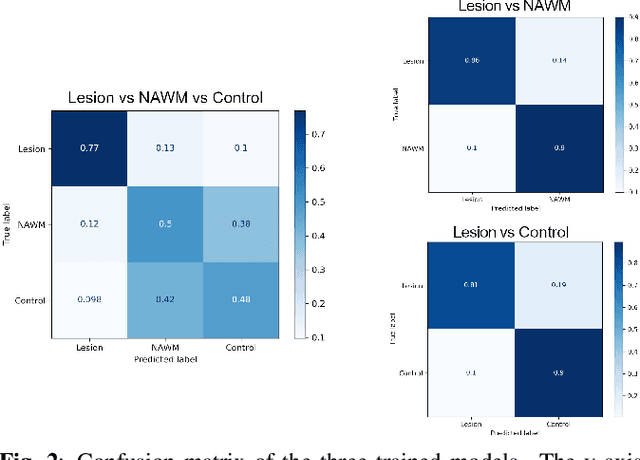
Abstract:Multiple sclerosis (MS) is an inflammatory and neurodegenerative disease characterized by diffuse and focal areas of tissue loss. Conventional MRI techniques such as T1-weighted and T2-weighted scans are generally used in the diagnosis and prognosis of the disease. Yet, these methods are limited by the lack of specificity between lesions, their perilesional area and non-lesional tissue. Alternative MRI techniques exhibit a higher level of sensitivity to focal and diffuse MS pathology than conventional MRI acquisitions. However, they still suffer from limited specificity when considered alone. In this work, we have combined tissue microstructure information derived from multicompartment diffusion MRI and T2 relaxometry models to explore the voxel-based prediction power of a machine learning model in a cohort of MS patients and healthy controls. Our results show that the combination of multi-modal features, together with a boosting enhanced decision-tree based classifier, which combines a set of weak classifiers to form a strong classifier via a voting mechanism, is able to utilise the complementary information for the classification of abnormal tissue.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge