Tobias Knopp
Automating Parameter Selection in Deep Image Prior for Fluorescence Microscopy Image Denoising via Similarity-Based Parameter Transfer
Jan 17, 2026Abstract:Unsupervised deep image prior (DIP) addresses shortcomings of training data requirements and limited generalization associated with supervised deep learning. The performance of DIP depends on the network architecture and the stopping point of its iterative process. Optimizing these parameters for a new image requires time, restricting DIP application in domains where many images need to be processed. Focusing on fluorescence microscopy data, we hypothesize that similar images share comparable optimal parameter configurations for DIP-based denoising, potentially enabling optimization-free DIP for fluorescence microscopy. We generated a calibration (n=110) and validation set (n=55) of semantically different images from an open-source dataset for a network architecture search targeted towards ideal U-net architectures and stopping points. The calibration set represented our transfer basis. The validation set enabled the assessment of which image similarity criterion yields the best results. We then implemented AUTO-DIP, a pipeline for automatic parameter transfer, and compared it to the originally published DIP configuration (baseline) and a state-of-the-art image-specific variational denoising approach. We show that a parameter transfer from the calibration dataset to a test image based on only image metadata similarity (e.g., microscope type, imaged specimen) leads to similar and better performance than a transfer based on quantitative image similarity measures. AUTO-DIP outperforms the baseline DIP (DIP with original DIP parameters) as well as the variational denoising approaches for several open-source test datasets of varying complexity, particularly for very noisy inputs. Applications to locally acquired fluorescence microscopy images further proved superiority of AUTO-DIP.
Efficient Chebyshev Reconstruction for the Anisotropic Equilibrium Model in Magnetic Particle Imaging
Apr 17, 2025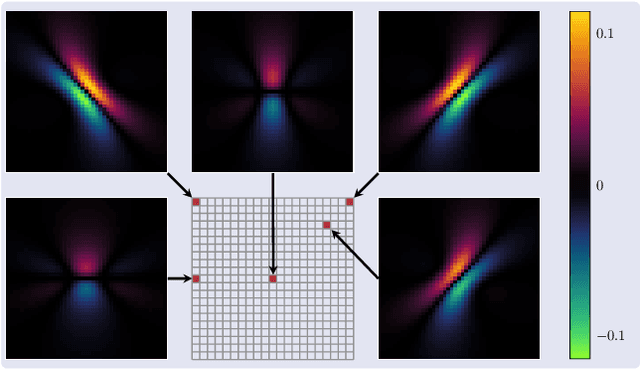
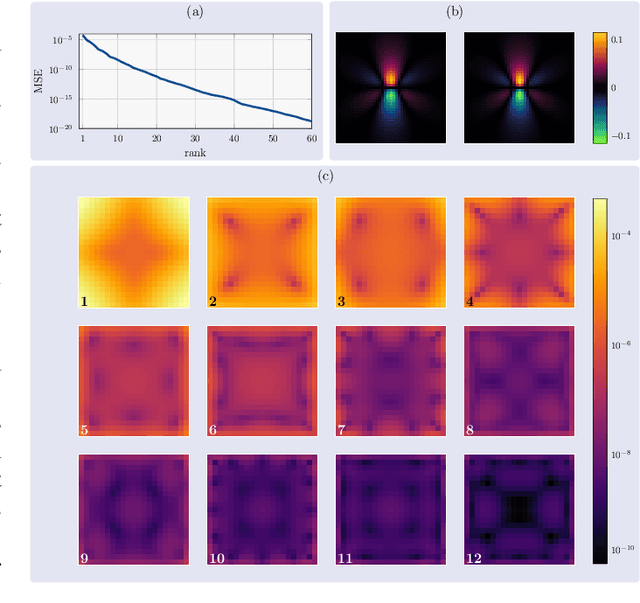
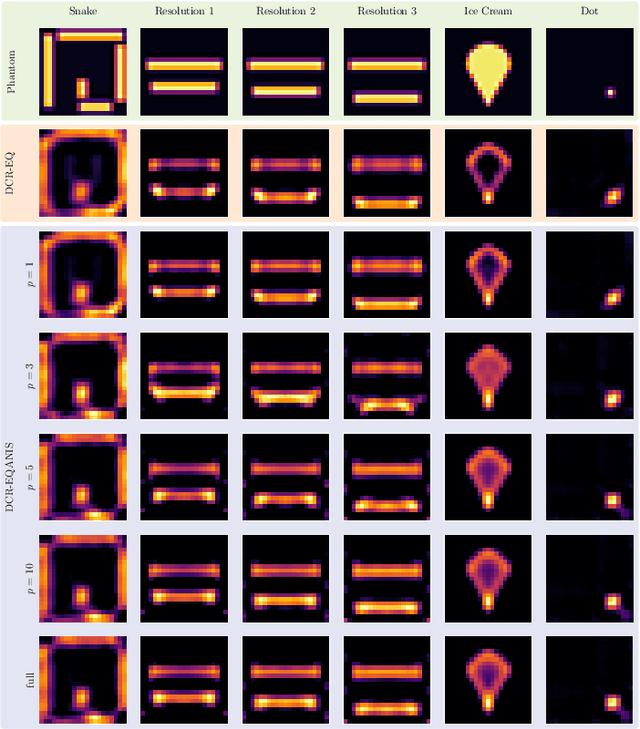
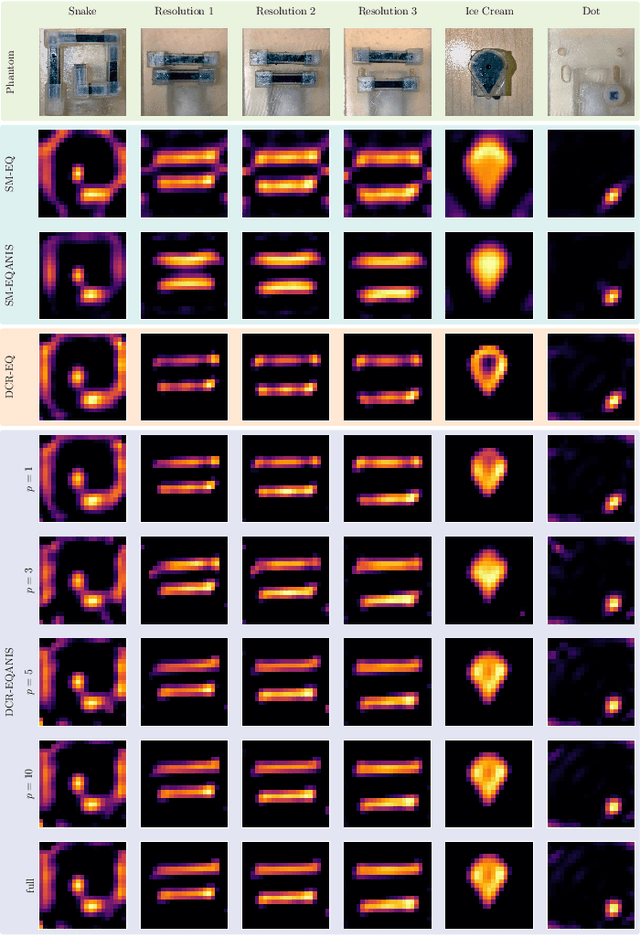
Abstract:Magnetic Particle Imaging (MPI) is a tomographic imaging modality capable of real-time, high-sensitivity mapping of superparamagnetic iron oxide nanoparticles. Model-based image reconstruction provides an alternative to conventional methods that rely on a measured system matrix, eliminating the need for laborious calibration measurements. Nevertheless, model-based approaches must account for the complexities of the imaging chain to maintain high image quality. A recently proposed direct reconstruction method leverages weighted Chebyshev polynomials in the frequency domain, removing the need for a simulated system matrix. However, the underlying model neglects key physical effects, such as nanoparticle anisotropy, leading to distortions in reconstructed images. To mitigate these artifacts, an adapted direct Chebyshev reconstruction (DCR) method incorporates a spatially variant deconvolution step, significantly improving reconstruction accuracy at the cost of increased computational demands. In this work, we evaluate the adapted DCR on six experimental phantoms, demonstrating enhanced reconstruction quality in real measurements and achieving image fidelity comparable to or exceeding that of simulated system matrix reconstruction. Furthermore, we introduce an efficient approximation for the spatially variable deconvolution, reducing both runtime and memory consumption while maintaining accuracy. This method achieves computational complexity of O(N log N ), making it particularly beneficial for high-resolution and three-dimensional imaging. Our results highlight the potential of the adapted DCR approach for improving model-based MPI reconstruction in practical applications.
Low-cost analog signal chain for transmit-receive circuits of passive induction-based resonators
Feb 05, 2025


Abstract:Passive wireless sensors are crucial in modern medical and industrial settings to monitor procedures and conditions. We demonstrate a circuit to inductively excite passive resonators and to conduct their decaying signal response to a low noise amplifier. Two design variations of a generic transmit-receive signal chain are proposed, measured, and described in detail for the purpose of facilitating replication. Instrumentation and design aim to be scalable for multi-channel array configurations, using either off-the-shelf class-D audio amplifiers or a custom full H-bridge. Measurements are conducted on miniature magneto-mechanical resonators in the ultra low frequency range to enable sensing and tracking applications of such devices in different environments.
Learned Discrepancy Reconstruction and Benchmark Dataset for Magnetic Particle Imaging
Jan 09, 2025



Abstract:Magnetic Particle Imaging (MPI) is an emerging imaging modality based on the magnetic response of superparamagnetic iron oxide nanoparticles to achieve high-resolution and real-time imaging without harmful radiation. One key challenge in the MPI image reconstruction task arises from its underlying noise model, which does not fulfill the implicit Gaussian assumptions that are made when applying traditional reconstruction approaches. To address this challenge, we introduce the Learned Discrepancy Approach, a novel learning-based reconstruction method for inverse problems that includes a learned discrepancy function. It enhances traditional techniques by incorporating an invertible neural network to explicitly model problem-specific noise distributions. This approach does not rely on implicit Gaussian noise assumptions, making it especially suited to handle the sophisticated noise model in MPI and also applicable to other inverse problems. To further advance MPI reconstruction techniques, we introduce the MPI-MNIST dataset - a large collection of simulated MPI measurements derived from the MNIST dataset of handwritten digits. The dataset includes noise-perturbed measurements generated from state-of-the-art model-based system matrices and measurements of a preclinical MPI scanner device. This provides a realistic and flexible environment for algorithm testing. Validated against the MPI-MNIST dataset, our method demonstrates significant improvements in reconstruction quality in terms of structural similarity when compared to classical reconstruction techniques.
Equilibrium Model with Anisotropy for Model-Based Reconstruction in Magnetic Particle Imaging
Mar 01, 2024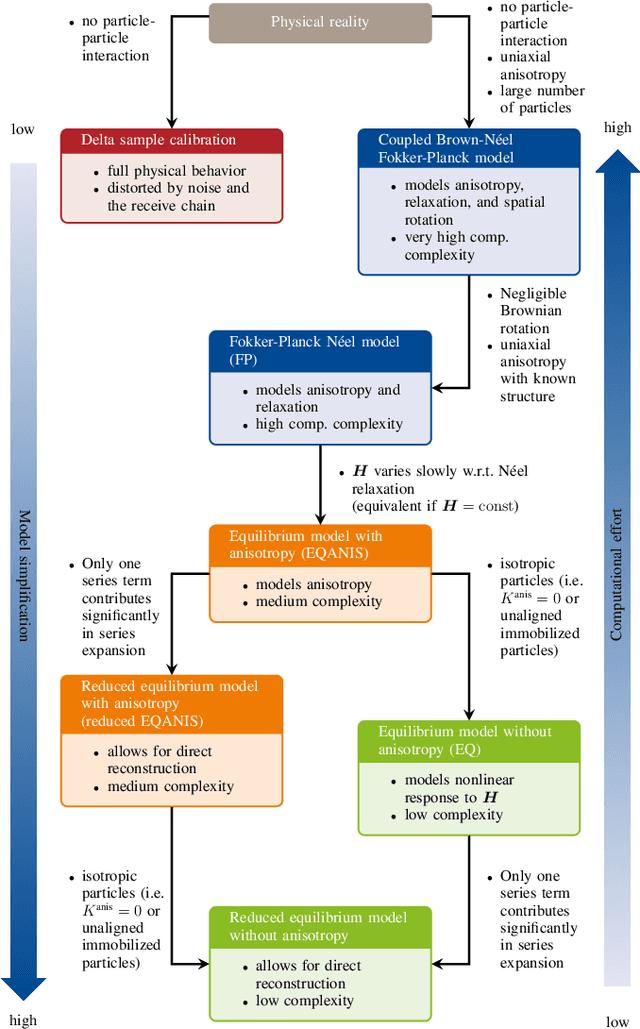
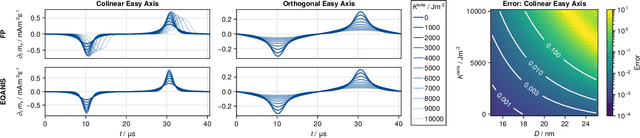
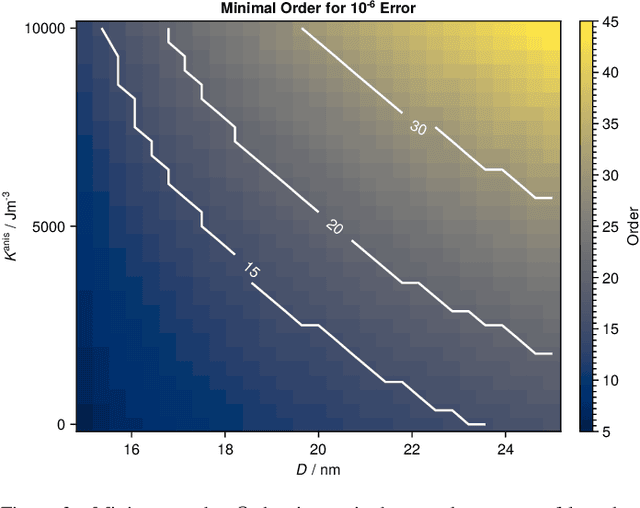
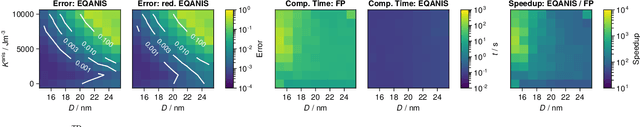
Abstract:Magnetic particle imaging is a tracer-based tomographic imaging technique that allows the concentration of magnetic nanoparticles to be determined with high spatio-temporal resolution. To reconstruct an image of the tracer concentration, the magnetization dynamics of the particles must be accurately modeled. A popular ensemble model is based on solving the Fokker-Plank equation, taking into account either Brownian or N\'eel dynamics. The disadvantage of this model is that it is computationally expensive due to an underlying stiff differential equation. A simplified model is the equilibrium model, which can be evaluated directly but in most relevant cases it suffers from a non-negligible modeling error. In the present work, we investigate an extended version of the equilibrium model that can account for particle anisotropy. We show that this model can be expressed as a series of Bessel functions, which can be truncated based on a predefined accuracy, leading to very short computation times, which are about three orders of magnitude lower than equivalent Fokker-Planck computation times. We investigate the accuracy of the model for 2D Lissajous MPI sequences and show that the difference between the Fokker-Planck and the equilibrium model with anisotropy is sufficiently small so that the latter model can be used for image reconstruction on experimental data with only marginal loss of image quality, even compared to a system matrix-based reconstruction.
Resonant Inductive Coupling Network for Human-Sized Magnetic Particle Imaging
Dec 23, 2023Abstract:In Magnetic Particle Imaging, a field-free region is maneuvered throughout the field of view using a time-varying magnetic field known as the drive-field. Human-sized systems operate the drive-field in the kHz range and generate it by utilizing strong currents that can rise to the kA range within a coil called the drive field generator. Matching and tuning between a power amplifier, a band-pass filter and the drive-field generator is required. Here, for reasons of safety in future human scanners, a symmetrical topology and a transformer, called inductive coupling network is used. Our primary objectives are to achieve floating potentials to ensure patient safety, attaining high linearity and high gain for the resonant transformer. We present a novel systematic approach to the design of a loss-optimized resonant toroid with a D-shaped cross section, employing segmentation to adjust the inductance-to-resistance ratio while maintaining a constant quality factor. Simultaneously, we derive a specific matching condition of a symmetric transmit-receive circuit for magnetic particle imaging. The chosen setup filters the fundamental frequency and allows simultaneous signal transmission and reception. In addition, the decoupling of multiple drive field channels is discussed and the primary side of the transformer is evaluated for maximum coupling and minimum stray field. Two prototypes were constructed, measured, decoupled, and compared to the derived theory and to method-of-moment based simulations.
Generalized MPI Multi-Patch Reconstruction using Clusters of similar System Matrices
May 02, 2022


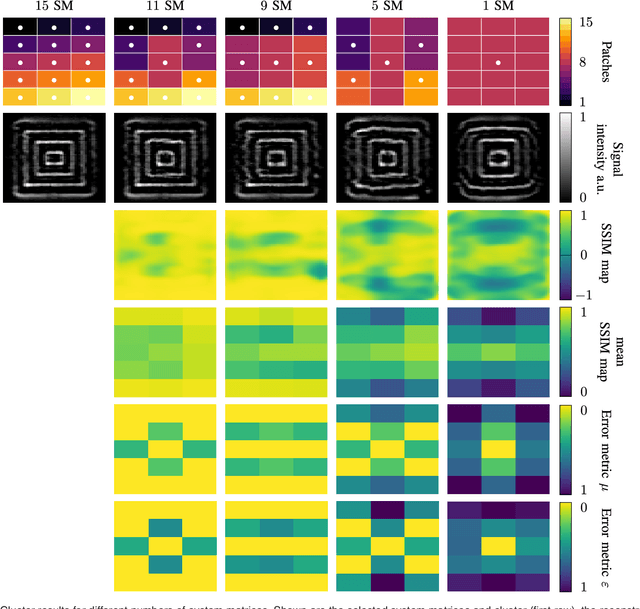
Abstract:The tomographic imaging method magnetic particle imaging (MPI) requires a multi-patch approach for capturing large field of views. This approach consists of a continuous or stepwise spatial shift of a small sub-volume of only few cubic centimeters size, which is scanned using one or multiple excitation fields in the kHz range. Under the assumption of ideal magnetic fields, the MPI system matrix is shift invariant and in turn a single matrix suffices for image reconstruction significantly reducing the calibration time and reconstruction effort. For large field imperfections, however, the method can lead to severe image artifacts. In the present work we generalize the efficient multi-patch reconstruction to work under non-ideal field conditions, where shift invariance holds only approximately for small shifts of the sub-volume. Patches are clustered based on a magnetic-field-based metric such that in each cluster the shift invariance holds in good approximation. The total number of clusters is the main parameter of our method and allows to trade off calibration time and image artifacts. The magnetic-field-based metric allows to perform the clustering without prior knowledge of the system matrices. The developed reconstruction algorithm is evaluated on a multi-patch measurement sequence with 15 patches, where efficient multi-patch reconstruction with a single calibration measurement leads to strong image artifacts. Analysis reveals that calibration measurements can be decreased from 15 to 11 with no visible image artifacts. A further reduction to 9 is possible with only slight degradation in image quality.
System Matrix based Reconstruction for Pulsed Sequences in Magnetic Particle Imaging
Aug 23, 2021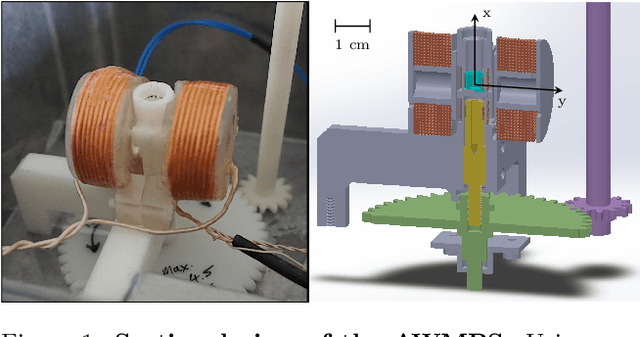
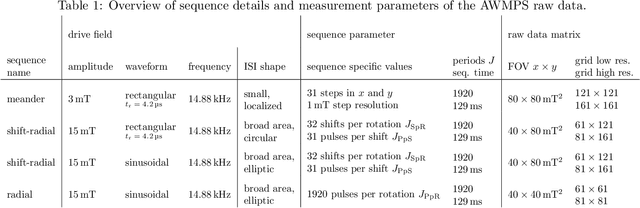
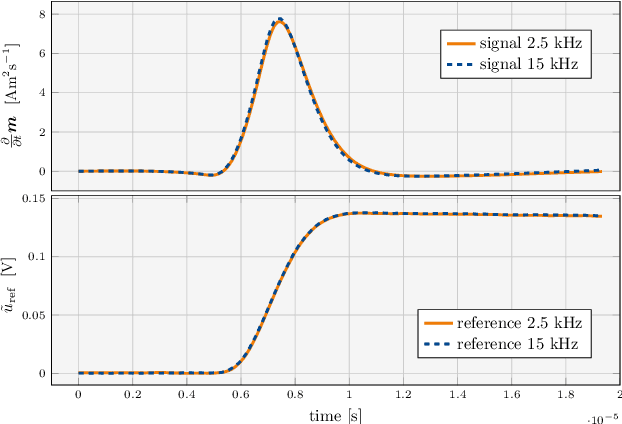
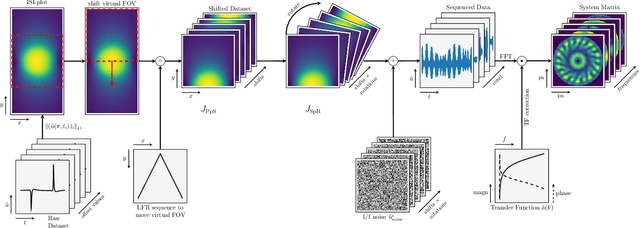
Abstract:Improving resolution and sensitivity will widen possible medical applications of magnetic particle imaging in its clinical application. Pulsed excitation promises such benefits, at the cost of more complex hardware solutions and restrictions on drive field amplitude and frequency. In this work, a sequence is proposed, that combines high drive-field amplitudes and high frequency rectangular excitation. State of the art systems utilize a sinusoidal excitation to drive superparamagnetic nanoparticles into the non-linear part of their magnetization curve, which creates a spectrum with a clear separation of direct feed-through and higher harmonics caused by the particles response. One challenge for rectangular excitation is the discrimination of particle and excitation signals, both broad-band. Another is the drive-field sequence itself, as particles that are not placed at the same spatial position, may react simultaneously and are not separable by their signals phase or signal shape. This loss of information in spatial encoding is overcome in this work by utilizing a superposition of shifting fields and drive-field rotations. The software framework developed for this work processes measured data from an Arbitrary Waveform Magnetic Particle Spectrometer, which is calibrated to guarantee device independence. Multiple sequence types and waveforms are compared, based on frequency space image reconstruction from emulated signals, that are derived from these measured particle responses. A resolution of 1.0 mT (0.8 mm for a gradient of (-1.25,-1.25,2.5) T/m) in x- and y-direction was achieved and a superior sensitivity was detected on the basis of reference phantoms for the proposed sequence in this work.
Intelligent Chest X-ray Worklist Prioritization by CNNs: A Clinical Workflow Simulation
Jan 23, 2020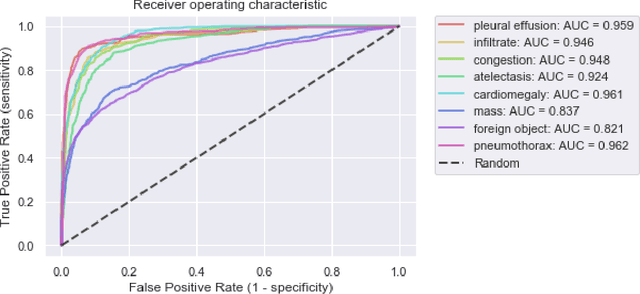
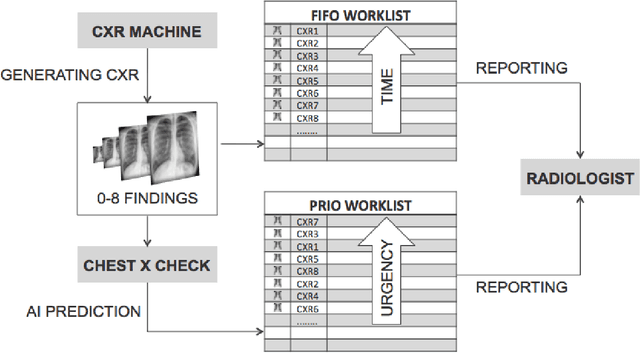
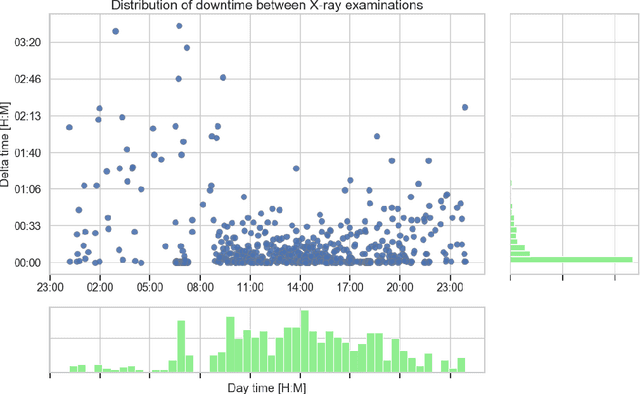
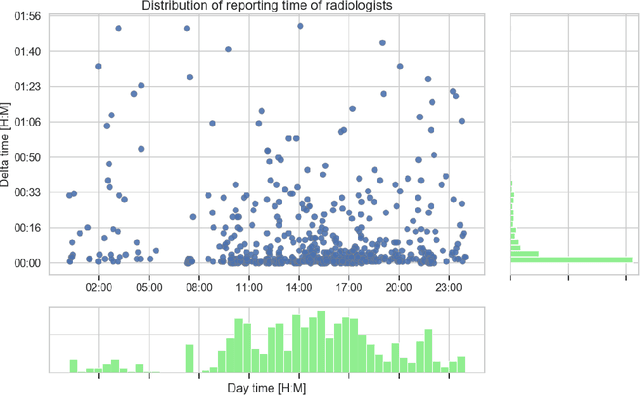
Abstract:Growing radiologic workload and shortage of medical experts worldwide often lead to delayed or even unreported examinations, which poses a risk for patient's safety in case of unrecognized findings in chest radiographs (CXR). The aim was to evaluate, whether deep learning algorithms for an intelligent worklist prioritization might optimize the radiology workflow and can reduce report turnaround times (RTAT) for critical findings, instead of reporting according to the First-In-First-Out-Principle (FIFO). Furthermore, we investigated the problem of false negative prediction in the context of worklist prioritization. To assess the potential benefit of an intelligent worklist prioritization, three different workflow simulations based on our analysis were run and RTAT were compared: FIFO (non-prioritized), Prio1 (prioritized) and Prio2 (prioritized, with RTATmax.). Examination triage was performed by "ChestXCheck", a convolutional neural network, classifying eight different pathological findings ranked in descending order of urgency: pneumothorax, pleural effusion, infiltrate, congestion, atelectasis, cardiomegaly, mass and foreign object. The average RTAT for all critical findings was significantly reduced by both Prio simulations compared to the FIFO simulation (e.g. pneumothorax: 32.1 min vs. 69.7 min; p < 0.0001), while the average RTAT for normal examinations increased at the same time (69.5 min vs. 90.0 min; p < 0.0001). Both effects were slightly lower at Prio2 than at Prio1, whereas the maximum RTAT at Prio1 was substantially higher for all classes, due to individual examinations rated false negative.Our Prio2 simulation demonstrated that intelligent worklist prioritization by deep learning algorithms reduces average RTAT for critical findings in chest X-ray while maintaining a similar maximum RTAT as FIFO.
3d-SMRnet: Achieving a new quality of MPI system matrix recovery by deep learning
May 08, 2019
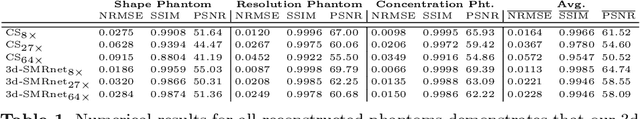
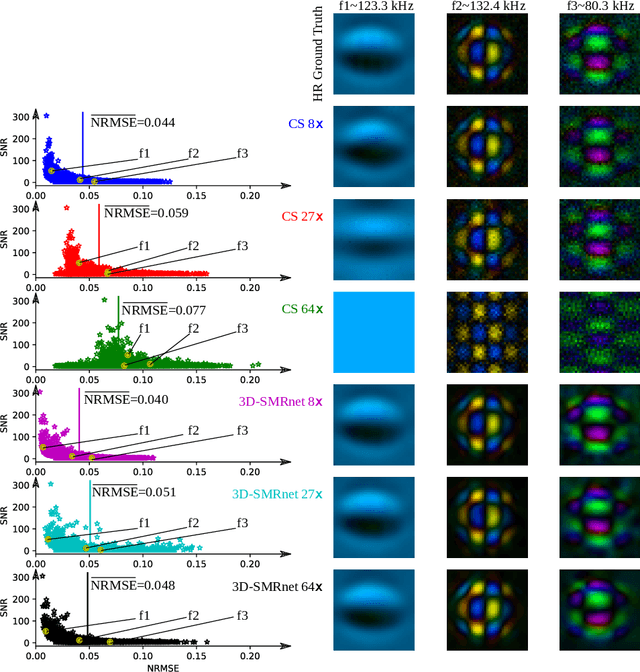
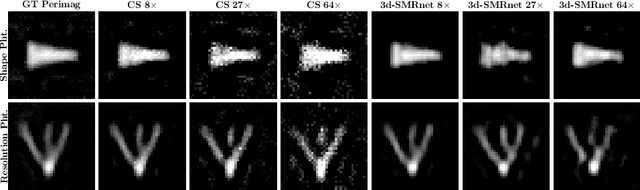
Abstract:Magnetic particle imaging (MPI) data is commonly reconstructed using a system matrix acquired in a time-consuming calibration measurement. The calibration approach has the important advantage over model-based reconstruction that it takes the complex particle physics as well as system imperfections into account. This benefit comes for the cost that the system matrix needs to be re-calibrated whenever the scan parameters, particle types or even the particle environment (e.g. viscosity or temperature) changes. One route for reducing the calibration time is the sampling of the system matrix at a subset of the spatial positions of the intended field-of-view and employing system matrix recovery. Recent approaches used compressed sensing (CS) and achieved subsampling factors up to 28 that still allowed reconstructing MPI images of sufficient quality. In this work, we propose a novel framework with a 3d-System Matrix Recovery Network and demonstrate it to recover a 3d system matrix with a subsampling factor of 64 in less than one minute and to outperform CS in terms of system matrix quality, reconstructed image quality, and processing time. The advantage of our method is demonstrated by reconstructing open access MPI datasets. The model is further shown to be capable of inferring system matrices for different particle types.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge