Temel Tirkes
Large-Scale Multi-Center CT and MRI Segmentation of Pancreas with Deep Learning
May 20, 2024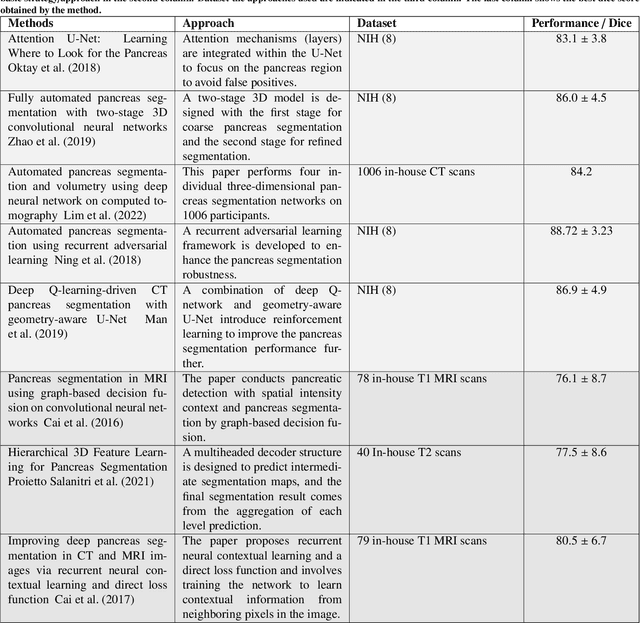
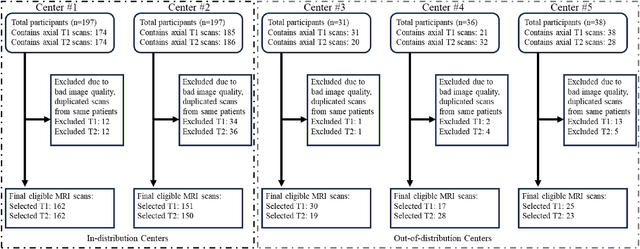
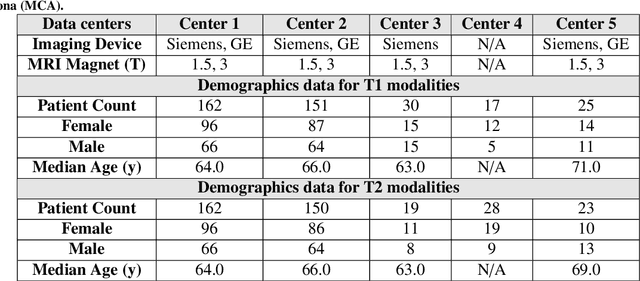
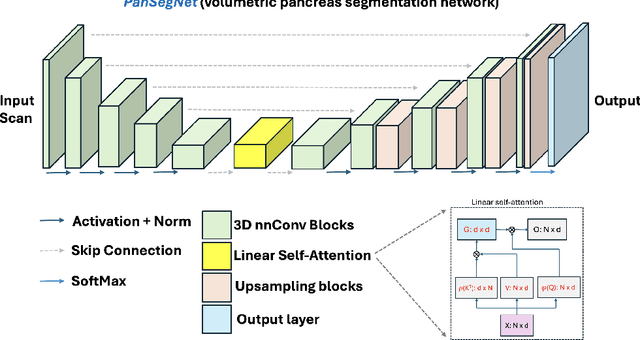
Abstract:Automated volumetric segmentation of the pancreas on cross-sectional imaging is needed for diagnosis and follow-up of pancreatic diseases. While CT-based pancreatic segmentation is more established, MRI-based segmentation methods are understudied, largely due to a lack of publicly available datasets, benchmarking research efforts, and domain-specific deep learning methods. In this retrospective study, we collected a large dataset (767 scans from 499 participants) of T1-weighted (T1W) and T2-weighted (T2W) abdominal MRI series from five centers between March 2004 and November 2022. We also collected CT scans of 1,350 patients from publicly available sources for benchmarking purposes. We developed a new pancreas segmentation method, called PanSegNet, combining the strengths of nnUNet and a Transformer network with a new linear attention module enabling volumetric computation. We tested PanSegNet's accuracy in cross-modality (a total of 2,117 scans) and cross-center settings with Dice and Hausdorff distance (HD95) evaluation metrics. We used Cohen's kappa statistics for intra and inter-rater agreement evaluation and paired t-tests for volume and Dice comparisons, respectively. For segmentation accuracy, we achieved Dice coefficients of 88.3% (std: 7.2%, at case level) with CT, 85.0% (std: 7.9%) with T1W MRI, and 86.3% (std: 6.4%) with T2W MRI. There was a high correlation for pancreas volume prediction with R^2 of 0.91, 0.84, and 0.85 for CT, T1W, and T2W, respectively. We found moderate inter-observer (0.624 and 0.638 for T1W and T2W MRI, respectively) and high intra-observer agreement scores. All MRI data is made available at https://osf.io/kysnj/. Our source code is available at https://github.com/NUBagciLab/PaNSegNet.
Detection of Peri-Pancreatic Edema using Deep Learning and Radiomics Techniques
Apr 25, 2024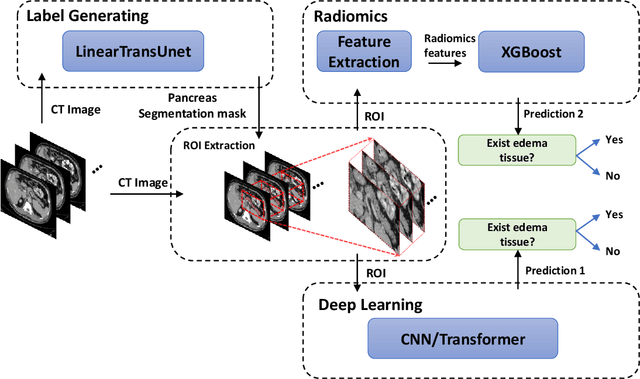
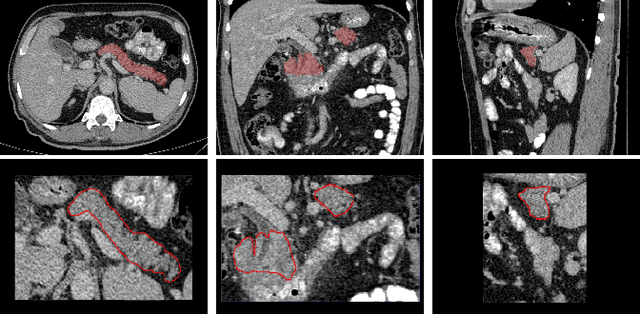
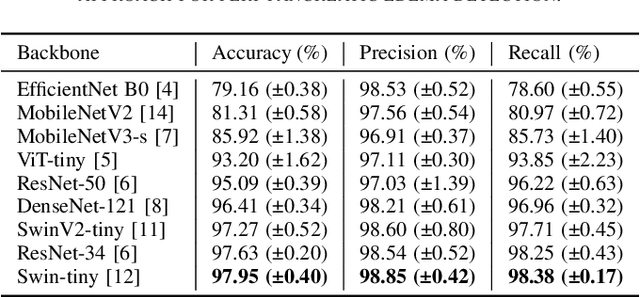
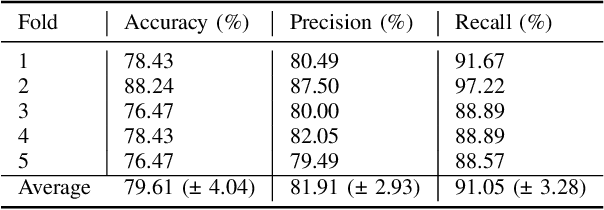
Abstract:Identifying peri-pancreatic edema is a pivotal indicator for identifying disease progression and prognosis, emphasizing the critical need for accurate detection and assessment in pancreatitis diagnosis and management. This study \textit{introduces a novel CT dataset sourced from 255 patients with pancreatic diseases, featuring annotated pancreas segmentation masks and corresponding diagnostic labels for peri-pancreatic edema condition}. With the novel dataset, we first evaluate the efficacy of the \textit{LinTransUNet} model, a linear Transformer based segmentation algorithm, to segment the pancreas accurately from CT imaging data. Then, we use segmented pancreas regions with two distinctive machine learning classifiers to identify existence of peri-pancreatic edema: deep learning-based models and a radiomics-based eXtreme Gradient Boosting (XGBoost). The LinTransUNet achieved promising results, with a dice coefficient of 80.85\%, and mIoU of 68.73\%. Among the nine benchmarked classification models for peri-pancreatic edema detection, \textit{Swin-Tiny} transformer model demonstrated the highest recall of $98.85 \pm 0.42$ and precision of $98.38\pm 0.17$. Comparatively, the radiomics-based XGBoost model achieved an accuracy of $79.61\pm4.04$ and recall of $91.05\pm3.28$, showcasing its potential as a supplementary diagnostic tool given its rapid processing speed and reduced training time. Our code is available \url{https://github.com/NUBagciLab/Peri-Pancreatic-Edema-Detection}.
Radiomics Boosts Deep Learning Model for IPMN Classification
Sep 11, 2023Abstract:Intraductal Papillary Mucinous Neoplasm (IPMN) cysts are pre-malignant pancreas lesions, and they can progress into pancreatic cancer. Therefore, detecting and stratifying their risk level is of ultimate importance for effective treatment planning and disease control. However, this is a highly challenging task because of the diverse and irregular shape, texture, and size of the IPMN cysts as well as the pancreas. In this study, we propose a novel computer-aided diagnosis pipeline for IPMN risk classification from multi-contrast MRI scans. Our proposed analysis framework includes an efficient volumetric self-adapting segmentation strategy for pancreas delineation, followed by a newly designed deep learning-based classification scheme with a radiomics-based predictive approach. We test our proposed decision-fusion model in multi-center data sets of 246 multi-contrast MRI scans and obtain superior performance to the state of the art (SOTA) in this field. Our ablation studies demonstrate the significance of both radiomics and deep learning modules for achieving the new SOTA performance compared to international guidelines and published studies (81.9\% vs 61.3\% in accuracy). Our findings have important implications for clinical decision-making. In a series of rigorous experiments on multi-center data sets (246 MRI scans from five centers), we achieved unprecedented performance (81.9\% accuracy).
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge