Shoji Kido
Visual Grounding of Whole Radiology Reports for 3D CT Images
Dec 08, 2023Abstract:Building a large-scale training dataset is an essential problem in the development of medical image recognition systems. Visual grounding techniques, which automatically associate objects in images with corresponding descriptions, can facilitate labeling of large number of images. However, visual grounding of radiology reports for CT images remains challenging, because so many kinds of anomalies are detectable via CT imaging, and resulting report descriptions are long and complex. In this paper, we present the first visual grounding framework designed for CT image and report pairs covering various body parts and diverse anomaly types. Our framework combines two components of 1) anatomical segmentation of images, and 2) report structuring. The anatomical segmentation provides multiple organ masks of given CT images, and helps the grounding model recognize detailed anatomies. The report structuring helps to accurately extract information regarding the presence, location, and type of each anomaly described in corresponding reports. Given the two additional image/report features, the grounding model can achieve better localization. In the verification process, we constructed a large-scale dataset with region-description correspondence annotations for 10,410 studies of 7,321 unique patients. We evaluated our framework using grounding accuracy, the percentage of correctly localized anomalies, as a metric and demonstrated that the combination of the anatomical segmentation and the report structuring improves the performance with a large margin over the baseline model (66.0% vs 77.8%). Comparison with the prior techniques also showed higher performance of our method.
* 14 pages, 7 figures. Accepted at MICCAI 2023
Large Batch and Patch Size Training for Medical Image Segmentation
Oct 24, 2022



Abstract:Multi-organ segmentation enables organ evaluation, accounts the relationship between multiple organs, and facilitates accurate diagnosis and treatment decisions. However, only few models can perform segmentation accurately because of the lack of datasets and computational resources. On AMOS2022 challenge, which is a large-scale, clinical, and diverse abdominal multiorgan segmentation benchmark, we trained a 3D-UNet model with large batch and patch sizes using multi-GPU distributed training. Segmentation performance tended to increase for models with large batch and patch sizes compared with the baseline settings. The accuracy was further improved by using ensemble models that were trained with different settings. These results provide a reference for parameter selection in organ segmentation.
Anatomy-aware Self-supervised Learning for Anomaly Detection in Chest Radiographs
May 09, 2022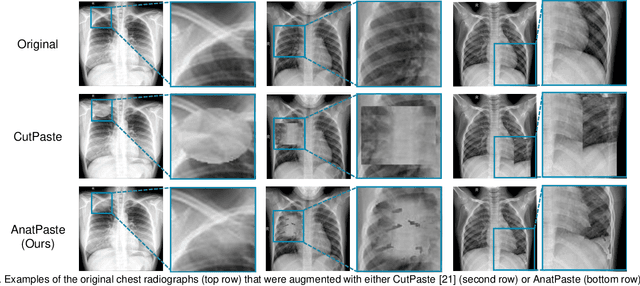
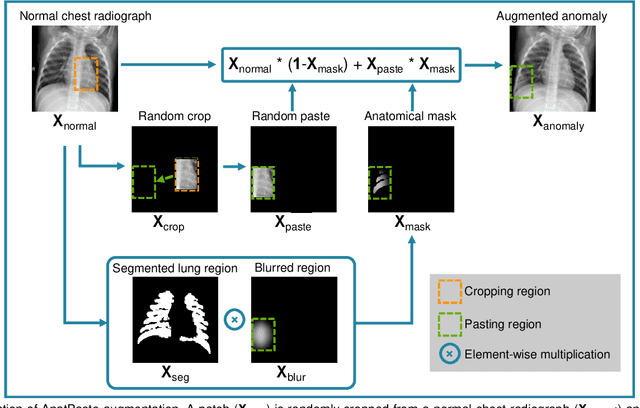
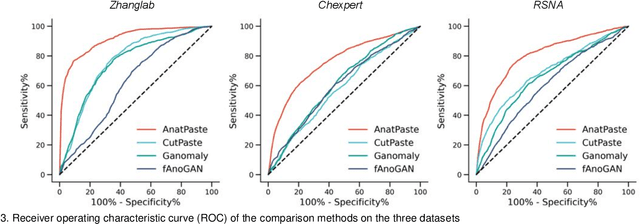
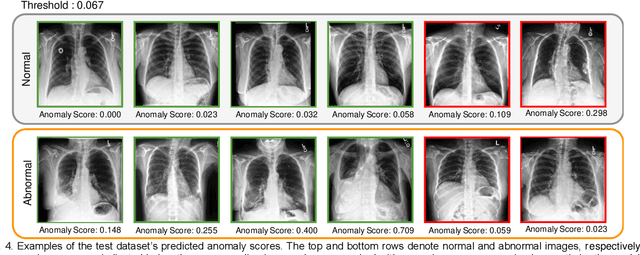
Abstract:Large numbers of labeled medical images are essential for the accurate detection of anomalies, but manual annotation is labor-intensive and time-consuming. Self-supervised learning (SSL) is a training method to learn data-specific features without manual annotation. Several SSL-based models have been employed in medical image anomaly detection. These SSL methods effectively learn representations in several field-specific images, such as natural and industrial product images. However, owing to the requirement of medical expertise, typical SSL-based models are inefficient in medical image anomaly detection. We present an SSL-based model that enables anatomical structure-based unsupervised anomaly detection (UAD). The model employs the anatomy-aware pasting (AnatPaste) augmentation tool. AnatPaste employs a threshold-based lung segmentation pretext task to create anomalies in normal chest radiographs, which are used for model pretraining. These anomalies are similar to real anomalies and help the model recognize them. We evaluate our model on three opensource chest radiograph datasets. Our model exhibit area under curves (AUC) of 92.1%, 78.7%, and 81.9%, which are the highest among existing UAD models. This is the first SSL model to employ anatomical information as a pretext task. AnatPaste can be applied in various deep learning models and downstream tasks. It can be employed for other modalities by fixing appropriate segmentation. Our code is publicly available at: https://github.com/jun-sato/AnatPaste.
Weak Supervision in Convolutional Neural Network for Semantic Segmentation of Diffuse Lung Diseases Using Partially Annotated Dataset
Mar 26, 2020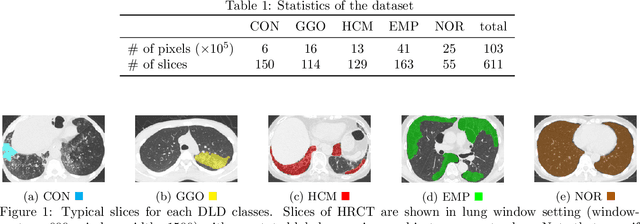
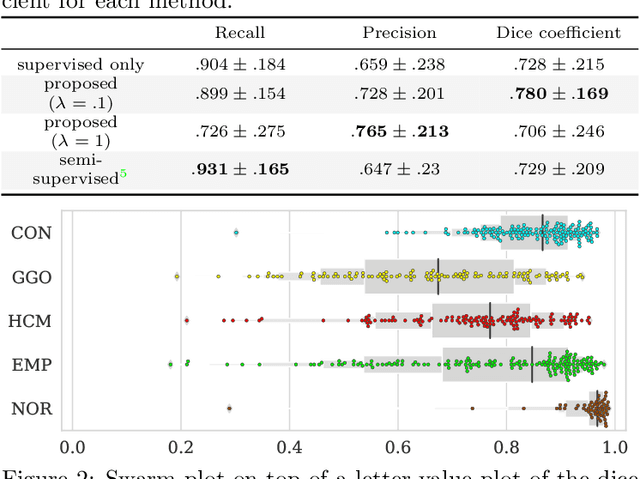
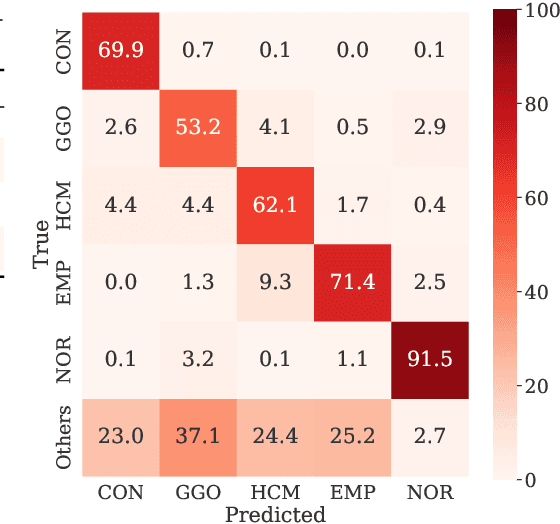
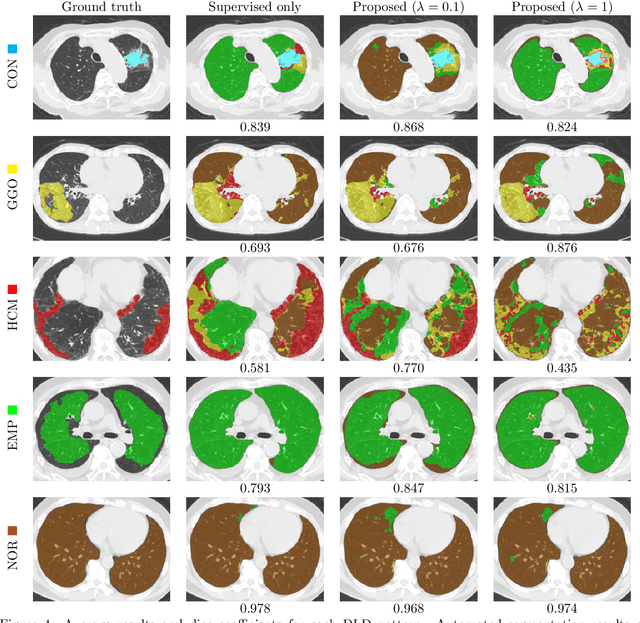
Abstract:Computer-aided diagnosis system for diffuse lung diseases (DLDs) is necessary for the objective assessment of the lung diseases. In this paper, we develop semantic segmentation model for 5 kinds of DLDs. DLDs considered in this work are consolidation, ground glass opacity, honeycombing, emphysema, and normal. Convolutional neural network (CNN) is one of the most promising technique for semantic segmentation among machine learning algorithms. While creating annotated dataset for semantic segmentation is laborious and time consuming, creating partially annotated dataset, in which only one chosen class is annotated for each image, is easier since annotators only need to focus on one class at a time during the annotation task. In this paper, we propose a new weak supervision technique that effectively utilizes partially annotated dataset. The experiments using partially annotated dataset composed 372 CT images demonstrated that our proposed technique significantly improved segmentation accuracy.
Feature Representation Analysis of Deep Convolutional Neural Network using Two-stage Feature Transfer -An Application for Diffuse Lung Disease Classification-
Oct 15, 2018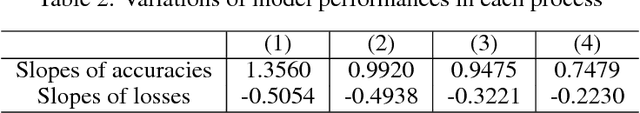
Abstract:Transfer learning is a machine learning technique designed to improve generalization performance by using pre-trained parameters obtained from other learning tasks. For image recognition tasks, many previous studies have reported that, when transfer learning is applied to deep neural networks, performance improves, despite having limited training data. This paper proposes a two-stage feature transfer learning method focusing on the recognition of textural medical images. During the proposed method, a model is successively trained with massive amounts of natural images, some textural images, and the target images. We applied this method to the classification task of textural X-ray computed tomography images of diffuse lung diseases. In our experiment, the two-stage feature transfer achieves the best performance compared to a from-scratch learning and a conventional single-stage feature transfer. We also investigated the robustness of the target dataset, based on size. Two-stage feature transfer shows better robustness than the other two learning methods. Moreover, we analyzed the feature representations obtained from DLDs imagery inputs for each feature transfer models using a visualization method. We showed that the two-stage feature transfer obtains both edge and textural features of DLDs, which does not occur in conventional single-stage feature transfer models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge