Shanshan Shan
SUSEP-Net: Simulation-Supervised and Contrastive Learning-based Deep Neural Networks for Susceptibility Source Separation
Jun 16, 2025Abstract:Quantitative susceptibility mapping (QSM) provides a valuable tool for quantifying susceptibility distributions in human brains; however, two types of opposing susceptibility sources (i.e., paramagnetic and diamagnetic), may coexist in a single voxel, and cancel each other out in net QSM images. Susceptibility source separation techniques enable the extraction of sub-voxel information from QSM maps. This study proposes a novel SUSEP-Net for susceptibility source separation by training a dual-branch U-net with a simulation-supervised training strategy. In addition, a contrastive learning framework is included to explicitly impose similarity-based constraints between the branch-specific guidance features in specially-designed encoders and the latent features in the decoders. Comprehensive experiments were carried out on both simulated and in vivo data, including healthy subjects and patients with pathological conditions, to compare SUSEP-Net with three state-of-the-art susceptibility source separation methods (i.e., APART-QSM, \c{hi}-separation, and \c{hi}-sepnet). SUSEP-Net consistently showed improved results compared with the other three methods, with better numerical metrics, improved high-intensity hemorrhage and calcification lesion contrasts, and reduced artifacts in brains with pathological conditions. In addition, experiments on an agarose gel phantom data were conducted to validate the accuracy and the generalization capability of SUSEP-Net.
Highly Undersampled MRI Reconstruction via a Single Posterior Sampling of Diffusion Models
May 13, 2025Abstract:Incoherent k-space under-sampling and deep learning-based reconstruction methods have shown great success in accelerating MRI. However, the performance of most previous methods will degrade dramatically under high acceleration factors, e.g., 8$\times$ or higher. Recently, denoising diffusion models (DM) have demonstrated promising results in solving this issue; however, one major drawback of the DM methods is the long inference time due to a dramatic number of iterative reverse posterior sampling steps. In this work, a Single Step Diffusion Model-based reconstruction framework, namely SSDM-MRI, is proposed for restoring MRI images from highly undersampled k-space. The proposed method achieves one-step reconstruction by first training a conditional DM and then iteratively distilling this model. Comprehensive experiments were conducted on both publicly available fastMRI images and an in-house multi-echo GRE (QSM) subject. Overall, the results showed that SSDM-MRI outperformed other methods in terms of numerical metrics (PSNR and SSIM), qualitative error maps, image fine details, and latent susceptibility information hidden in MRI phase images. In addition, the reconstruction time for a 320*320 brain slice of SSDM-MRI is only 0.45 second, which is only comparable to that of a simple U-net, making it a highly effective solution for MRI reconstruction tasks.
IR2QSM: Quantitative Susceptibility Mapping via Deep Neural Networks with Iterative Reverse Concatenations and Recurrent Modules
Jun 18, 2024Abstract:Quantitative susceptibility mapping (QSM) is an MRI phase-based post-processing technique to extract the distribution of tissue susceptibilities, demonstrating significant potential in studying neurological diseases. However, the ill-conditioned nature of dipole inversion makes QSM reconstruction from the tissue field prone to noise and artifacts. In this work, we propose a novel deep learning-based IR2QSM method for QSM reconstruction. It is designed by iterating four times of a reverse concatenations and middle recurrent modules enhanced U-net, which could dramatically improve the efficiency of latent feature utilization. Simulated and in vivo experiments were conducted to compare IR2QSM with several traditional algorithms (MEDI and iLSQR) and state-of-the-art deep learning methods (U-net, xQSM, and LPCNN). The results indicated that IR2QSM was able to obtain QSM images with significantly increased accuracy and mitigated artifacts over other methods. Particularly, IR2QSM demonstrated on average the best NRMSE (27.59%) in simulated experiments, which is 15.48%, 7.86%, 17.24%, 9.26%, and 29.13% lower than iLSQR, MEDI, U-net, xQSM, LPCNN, respectively, and led to improved QSM results with fewer artifacts for the in vivo data.
Image Reconstruction with B0 Inhomogeneity using an Interpretable Deep Unrolled Network on an Open-bore MRI-Linac
Apr 15, 2024



Abstract:MRI-Linac systems require fast image reconstruction with high geometric fidelity to localize and track tumours for radiotherapy treatments. However, B0 field inhomogeneity distortions and slow MR acquisition potentially limit the quality of the image guidance and tumour treatments. In this study, we develop an interpretable unrolled network, referred to as RebinNet, to reconstruct distortion-free images from B0 inhomogeneity-corrupted k-space for fast MRI-guided radiotherapy applications. RebinNet includes convolutional neural network (CNN) blocks to perform image regularizations and nonuniform fast Fourier Transform (NUFFT) modules to incorporate B0 inhomogeneity information. The RebinNet was trained on a publicly available MR dataset from eleven healthy volunteers for both fully sampled and subsampled acquisitions. Grid phantom and human brain images acquired from an open-bore 1T MRI-Linac scanner were used to evaluate the performance of the proposed network. The RebinNet was compared with the conventional regularization algorithm and our recently developed UnUNet method in terms of root mean squared error (RMSE), structural similarity (SSIM), residual distortions, and computation time. Imaging results demonstrated that the RebinNet reconstructed images with lowest RMSE (<0.05) and highest SSIM (>0.92) at four-time acceleration for simulated brain images. The RebinNet could better preserve structural details and substantially improve the computational efficiency (ten-fold faster) compared to the conventional regularization methods, and had better generalization ability than the UnUNet method. The proposed RebinNet can achieve rapid image reconstruction and overcome the B0 inhomogeneity distortions simultaneously, which would facilitate accurate and fast image guidance in radiotherapy treatments.
Plug-and-Play Latent Feature Editing for Orientation-Adaptive Quantitative Susceptibility Mapping Neural Networks
Nov 14, 2023Abstract:Quantitative susceptibility mapping (QSM) is a post-processing technique for deriving tissue magnetic susceptibility distribution from MRI phase measurements. Deep learning (DL) algorithms hold great potential for solving the ill-posed QSM reconstruction problem. However, a significant challenge facing current DL-QSM approaches is their limited adaptability to magnetic dipole field orientation variations during training and testing. In this work, we propose a novel Orientation-Adaptive Latent Feature Editing (OA-LFE) module to learn the encoding of acquisition orientation vectors and seamlessly integrate them into the latent features of deep networks. Importantly, it can be directly Plug-and-Play (PnP) into various existing DL-QSM architectures, enabling reconstructions of QSM from arbitrary magnetic dipole orientations. Its effectiveness is demonstrated by combining the OA-LFE module into our previously proposed phase-to-susceptibility single-step instant QSM (iQSM) network, which was initially tailored for pure-axial acquisitions. The proposed OA-LFE-empowered iQSM, which we refer to as iQSM+, is trained in a self-supervised manner on a specially-designed simulation brain dataset. Comprehensive experiments are conducted on simulated and in vivo human brain datasets, encompassing subjects ranging from healthy individuals to those with pathological conditions. These experiments involve various MRI platforms (3T and 7T) and aim to compare our proposed iQSM+ against several established QSM reconstruction frameworks, including the original iQSM. The iQSM+ yields QSM images with significantly improved accuracies and mitigates artifacts, surpassing other state-of-the-art DL-QSM algorithms.
Distortion-Corrected Image Reconstruction with Deep Learning on an MRI-Linac
May 23, 2022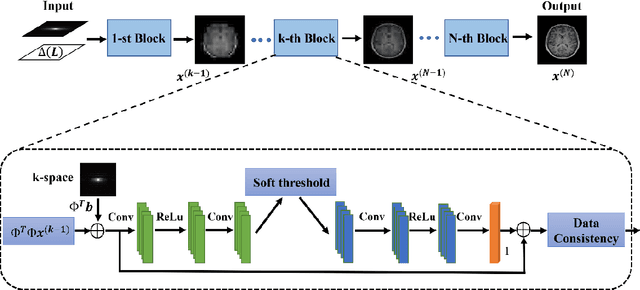
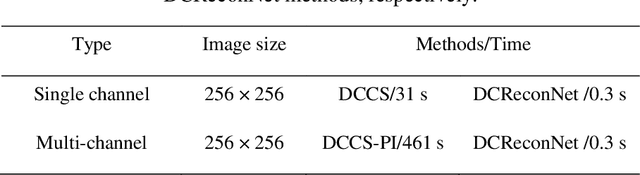
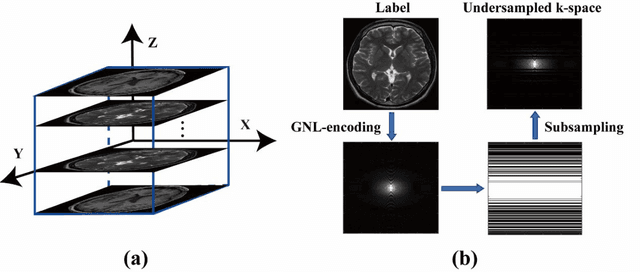
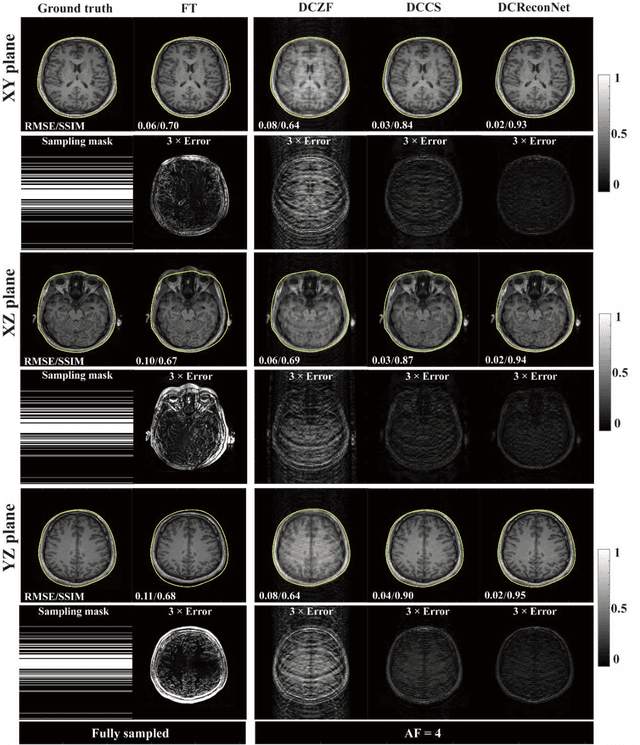
Abstract:Magnetic resonance imaging (MRI) is increasingly utilized for image-guided radiotherapy due to its outstanding soft-tissue contrast and lack of ionizing radiation. However, geometric distortions caused by gradient nonlinearity (GNL) limit anatomical accuracy, potentially compromising the quality of tumour treatments. In addition, slow MR acquisition and reconstruction limit the potential for real-time image guidance. Here, we demonstrate a deep learning-based method that rapidly reconstructs distortion-corrected images from raw k-space data for real-time MR-guided radiotherapy applications. We leverage recent advances in interpretable unrolling networks to develop a Distortion-Corrected Reconstruction Network (DCReconNet) that applies convolutional neural networks (CNNs) to learn effective regularizations and nonuniform fast Fourier transforms for GNL-encoding. DCReconNet was trained on a public MR brain dataset from eleven healthy volunteers for fully sampled and accelerated techniques including parallel imaging (PI) and compressed sensing (CS). The performance of DCReconNet was tested on phantom and volunteer brain data acquired on a 1.0T MRI-Linac. The DCReconNet, CS- and PI-based reconstructed image quality was measured by structural similarity (SSIM) and root-mean-squared error (RMSE) for numerical comparisons. The computation time for each method was also reported. Phantom and volunteer results demonstrated that DCReconNet better preserves image structure when compared to CS- and PI-based reconstruction methods. DCReconNet resulted in highest SSIM (0.95 median value) and lowest RMSE (<0.04) on simulated brain images with four times acceleration. DCReconNet is over 100-times faster than iterative, regularized reconstruction methods. DCReconNet provides fast and geometrically accurate image reconstruction and has potential for real-time MRI-guided radiotherapy applications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge