Sayantan Kumar
Forecasting from Clinical Textual Time Series: Adaptations of the Encoder and Decoder Language Model Families
Apr 14, 2025Abstract:Clinical case reports encode rich, temporal patient trajectories that are often underexploited by traditional machine learning methods relying on structured data. In this work, we introduce the forecasting problem from textual time series, where timestamped clinical findings--extracted via an LLM-assisted annotation pipeline--serve as the primary input for prediction. We systematically evaluate a diverse suite of models, including fine-tuned decoder-based large language models and encoder-based transformers, on tasks of event occurrence prediction, temporal ordering, and survival analysis. Our experiments reveal that encoder-based models consistently achieve higher F1 scores and superior temporal concordance for short- and long-horizon event forecasting, while fine-tuned masking approaches enhance ranking performance. In contrast, instruction-tuned decoder models demonstrate a relative advantage in survival analysis, especially in early prognosis settings. Our sensitivity analyses further demonstrate the importance of time ordering, which requires clinical time series construction, as compared to text ordering, the format of the text inputs that LLMs are classically trained on. This highlights the additional benefit that can be ascertained from time-ordered corpora, with implications for temporal tasks in the era of widespread LLM use.
Multimodal Variational Autoencoder: a Barycentric View
Dec 29, 2024Abstract:Multiple signal modalities, such as vision and sounds, are naturally present in real-world phenomena. Recently, there has been growing interest in learning generative models, in particular variational autoencoder (VAE), to for multimodal representation learning especially in the case of missing modalities. The primary goal of these models is to learn a modality-invariant and modality-specific representation that characterizes information across multiple modalities. Previous attempts at multimodal VAEs approach this mainly through the lens of experts, aggregating unimodal inference distributions with a product of experts (PoE), a mixture of experts (MoE), or a combination of both. In this paper, we provide an alternative generic and theoretical formulation of multimodal VAE through the lens of barycenter. We first show that PoE and MoE are specific instances of barycenters, derived by minimizing the asymmetric weighted KL divergence to unimodal inference distributions. Our novel formulation extends these two barycenters to a more flexible choice by considering different types of divergences. In particular, we explore the Wasserstein barycenter defined by the 2-Wasserstein distance, which better preserves the geometry of unimodal distributions by capturing both modality-specific and modality-invariant representations compared to KL divergence. Empirical studies on three multimodal benchmarks demonstrated the effectiveness of the proposed method.
Multimodal hierarchical multi-task deep learning framework for jointly predicting and explaining Alzheimer disease progression
Apr 04, 2024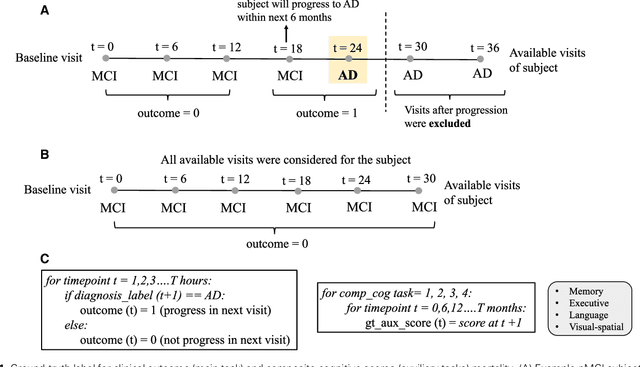
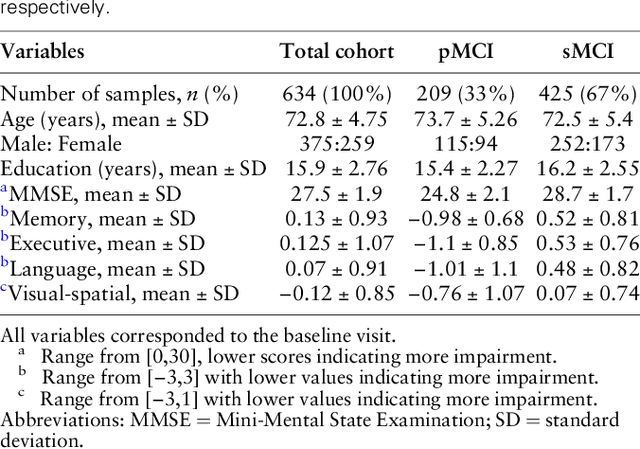
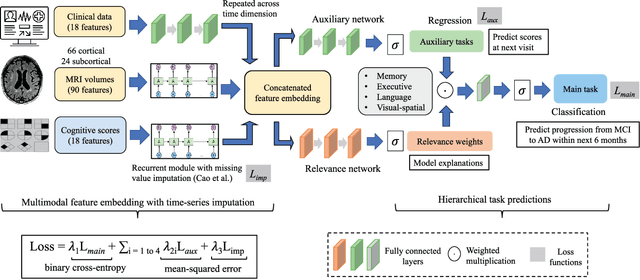
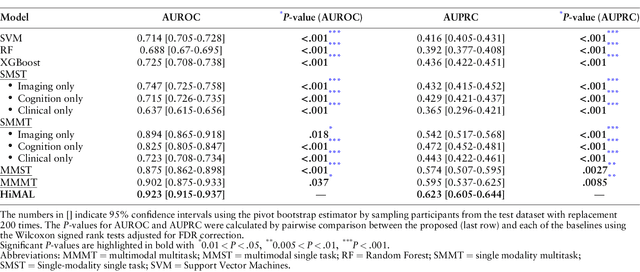
Abstract:Early identification of Mild Cognitive Impairment (MCI) subjects who will eventually progress to Alzheimer Disease (AD) is challenging. Existing deep learning models are mostly single-modality single-task models predicting risk of disease progression at a fixed timepoint. We proposed a multimodal hierarchical multi-task learning approach which can monitor the risk of disease progression at each timepoint of the visit trajectory. Longitudinal visit data from multiple modalities (MRI, cognition, and clinical data) were collected from MCI individuals of the Alzheimer Disease Neuroimaging Initiative (ADNI) dataset. Our hierarchical model predicted at every timepoint a set of neuropsychological composite cognitive function scores as auxiliary tasks and used the forecasted scores at every timepoint to predict the future risk of disease. Relevance weights for each composite function provided explanations about potential factors for disease progression. Our proposed model performed better than state-of-the-art baselines in predicting AD progression risk and the composite scores. Ablation study on the number of modalities demonstrated that imaging and cognition data contributed most towards the outcome. Model explanations at each timepoint can inform clinicians 6 months in advance the potential cognitive function decline that can lead to progression to AD in future. Our model monitored their risk of AD progression every 6 months throughout the visit trajectory of individuals. The hierarchical learning of auxiliary tasks allowed better optimization and allowed longitudinal explanations for the outcome. Our framework is flexible with the number of input modalities and the selection of auxiliary tasks and hence can be generalized to other clinical problems too.
AgileFormer: Spatially Agile Transformer UNet for Medical Image Segmentation
Mar 29, 2024



Abstract:In the past decades, deep neural networks, particularly convolutional neural networks, have achieved state-of-the-art performance in a variety of medical image segmentation tasks. Recently, the introduction of the vision transformer (ViT) has significantly altered the landscape of deep segmentation models. There has been a growing focus on ViTs, driven by their excellent performance and scalability. However, we argue that the current design of the vision transformer-based UNet (ViT-UNet) segmentation models may not effectively handle the heterogeneous appearance (e.g., varying shapes and sizes) of objects of interest in medical image segmentation tasks. To tackle this challenge, we present a structured approach to introduce spatially dynamic components to the ViT-UNet. This adaptation enables the model to effectively capture features of target objects with diverse appearances. This is achieved by three main components: \textbf{(i)} deformable patch embedding; \textbf{(ii)} spatially dynamic multi-head attention; \textbf{(iii)} deformable positional encoding. These components were integrated into a novel architecture, termed AgileFormer. AgileFormer is a spatially agile ViT-UNet designed for medical image segmentation. Experiments in three segmentation tasks using publicly available datasets demonstrated the effectiveness of the proposed method. The code is available at \href{https://github.com/sotiraslab/AgileFormer}{https://github.com/sotiraslab/AgileFormer}.
Improving Normative Modeling for Multi-modal Neuroimaging Data using mixture-of-product-of-experts variational autoencoders
Dec 02, 2023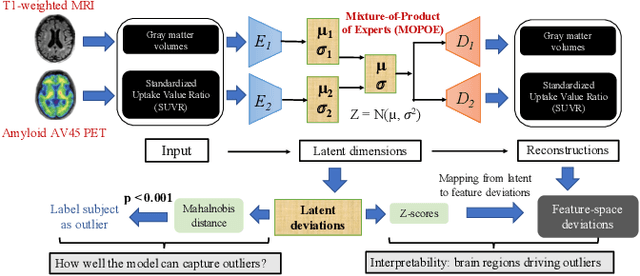
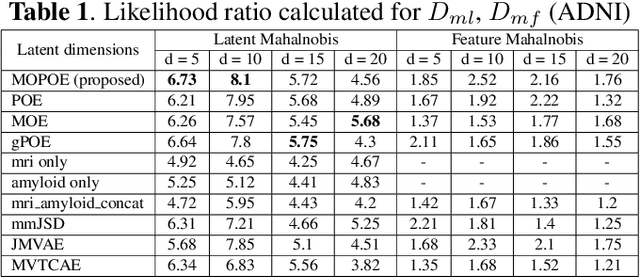
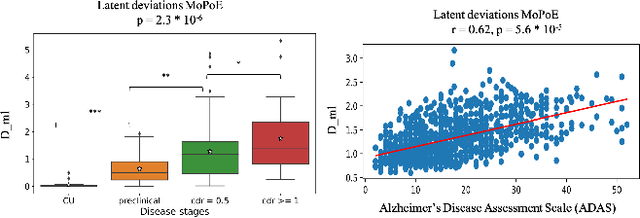
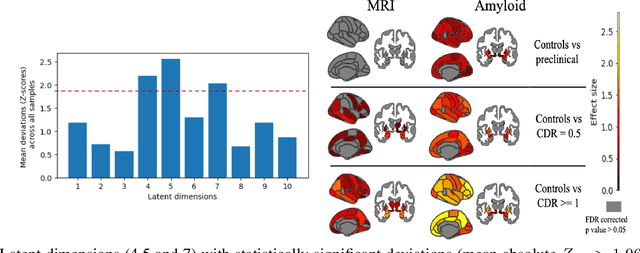
Abstract:Normative models in neuroimaging learn the brain patterns of healthy population distribution and estimate how disease subjects like Alzheimer's Disease (AD) deviate from the norm. Existing variational autoencoder (VAE)-based normative models using multimodal neuroimaging data aggregate information from multiple modalities by estimating product or averaging of unimodal latent posteriors. This can often lead to uninformative joint latent distributions which affects the estimation of subject-level deviations. In this work, we addressed the prior limitations by adopting the Mixture-of-Product-of-Experts (MoPoE) technique which allows better modelling of the joint latent posterior. Our model labelled subjects as outliers by calculating deviations from the multimodal latent space. Further, we identified which latent dimensions and brain regions were associated with abnormal deviations due to AD pathology.
Identifying Interpretable Clinical Subtypes withinHeterogeneous Dementia Clinic Population
Jan 31, 2022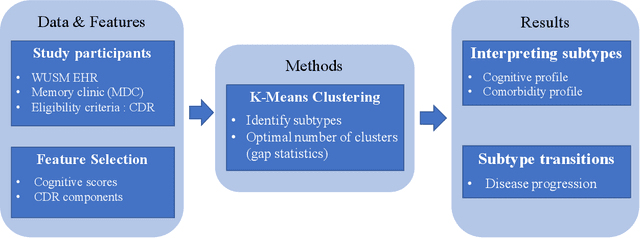
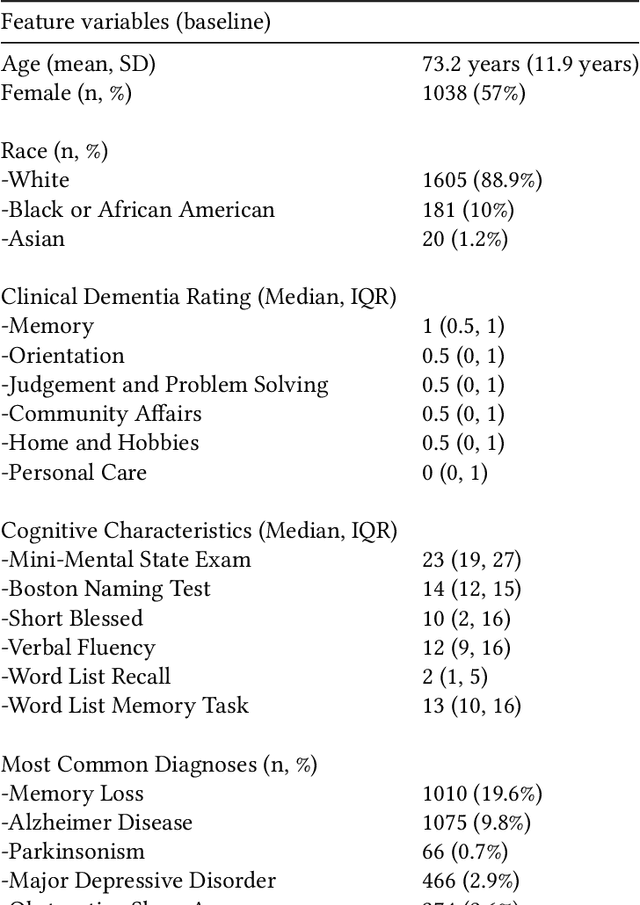
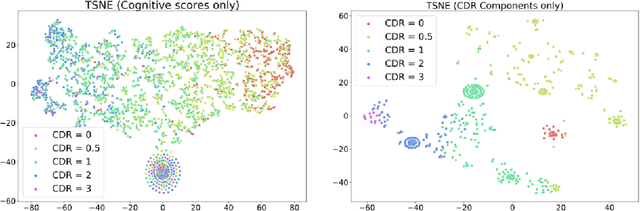

Abstract:Dementia is a highly heterogeneous neurodegenerative disorder. Differences in brain pathologies lead to significant variations in the clinical presentation and progression course of patients, increasing the need for individual progression predictions. Unsupervised cluster analysis on a dementia clinic population using the Clinical Dementia Rating (CDR) component scores uncovered subtypes with different risk of dementia progression. The distribution of the CDR components provide validation and interpretability regarding the cognitive characteristics of the identified subtypes.
NormVAE: Normative Modeling on Neuroimaging Data using Variational Autoencoders
Oct 10, 2021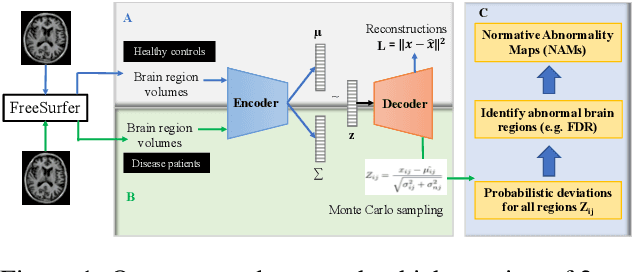

Abstract:Normative modeling is an emerging method for understanding the heterogeneous biology underlying neuropsychiatric and neurodegenerative disorders at the level of the individual participant. Deep autoencoders have been implemented as normative models, where patient-level deviations are modelled as the squared difference between the actual and reconstructed input without any uncertainty estimates in the deviations. In this study, we assessed NormVAE, a novel normative modeling based variational autoencoder (VAE) which calculates subject-level normative abnormality maps (NAM) for quantifying uncertainty in the deviations. Our experiments on brain neuroimaging data of Alzheimer's Disease (AD) patients demonstrated that the NormVAE-generated patient-level abnormality maps exhibit increased sensitivity to disease staging compared to a baseline VAE, which generates deterministic subject-level deviations without any uncertainty estimates.
Self-explaining Neural Network with Plausible Explanations
Oct 09, 2021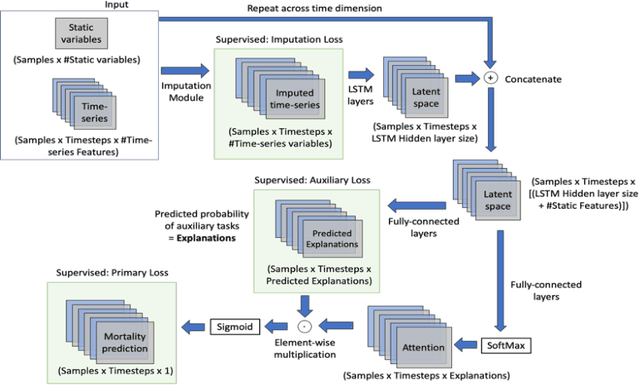
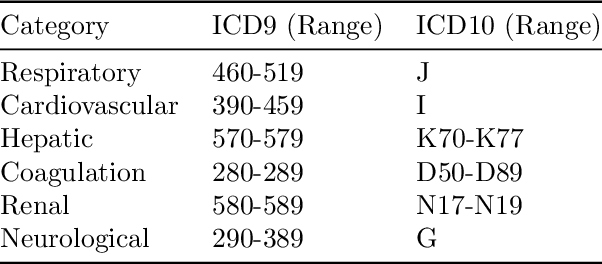
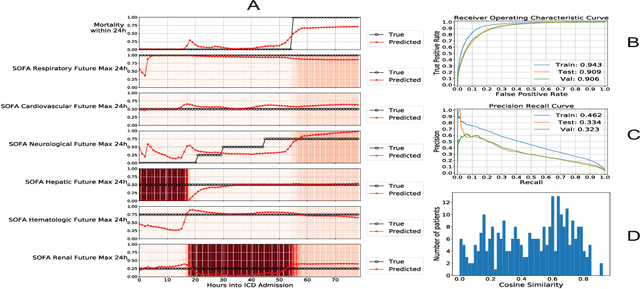
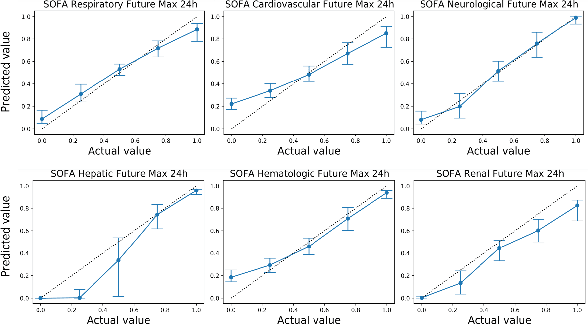
Abstract:Explaining the predictions of complex deep learning models, often referred to as black boxes, is critical in high-stakes domains like healthcare. However, post-hoc model explanations often are not understandable by clinicians and are difficult to integrate into clinical workflow. Further, while most explainable models use individual clinical variables as units of explanation, human understanding often rely on higher-level concepts or feature representations. In this paper, we propose a novel, self-explaining neural network for longitudinal in-hospital mortality prediction using domain-knowledge driven Sequential Organ Failure Assessment (SOFA) organ-specific scores as the atomic units of explanation. We also design a novel procedure to quantitatively validate the model explanations against gold standard discharge diagnosis information of patients. Our results provide interesting insights into how each of the SOFA organ scores contribute to mortality at different timesteps within longitudinal patient trajectory.
Machine learning for modeling the progression of Alzheimer disease dementia using clinical data: a systematic literature review
Aug 05, 2021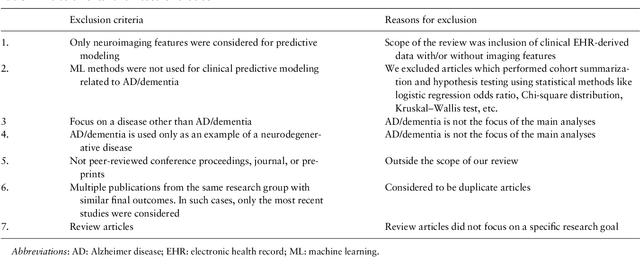
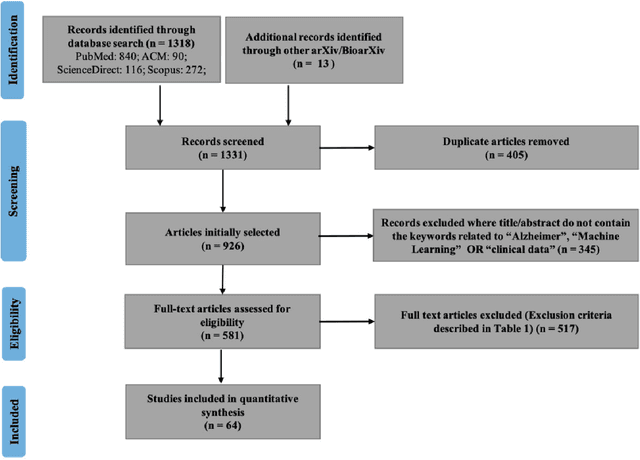
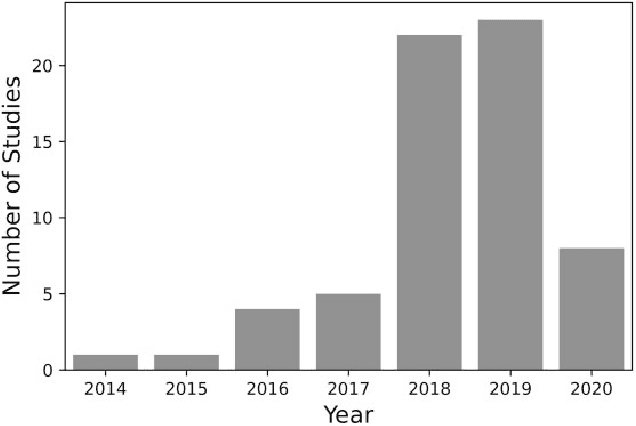

Abstract:Objective Alzheimer disease (AD) is the most common cause of dementia, a syndrome characterized by cognitive impairment severe enough to interfere with activities of daily life. We aimed to conduct a systematic literature review (SLR) of studies that applied machine learning (ML) methods to clinical data derived from electronic health records in order to model risk for progression of AD dementia. Materials and Methods We searched for articles published between January 1, 2010, and May 31, 2020, in PubMed, Scopus, ScienceDirect, IEEE Explore Digital Library, Association for Computing Machinery Digital Library, and arXiv. We used predefined criteria to select relevant articles and summarized them according to key components of ML analysis such as data characteristics, computational algorithms, and research focus. Results There has been a considerable rise over the past 5 years in the number of research papers using ML-based analysis for AD dementia modeling. We reviewed 64 relevant articles in our SLR. The results suggest that majority of existing research has focused on predicting progression of AD dementia using publicly available datasets containing both neuroimaging and clinical data (neurobehavioral status exam scores, patient demographics, neuroimaging data, and laboratory test values). Discussion Identifying individuals at risk for progression of AD dementia could potentially help to personalize disease management to plan future care. Clinical data consisting of both structured data tables and clinical notes can be effectively used in ML-based approaches to model risk for AD dementia progression. Data sharing and reproducibility of results can enhance the impact, adaptation, and generalizability of this research.
* 10 pages, 4 figures, 3 tables
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge