Pim Moeskops
Inferring a Third Spatial Dimension from 2D Histological Images
Jan 10, 2018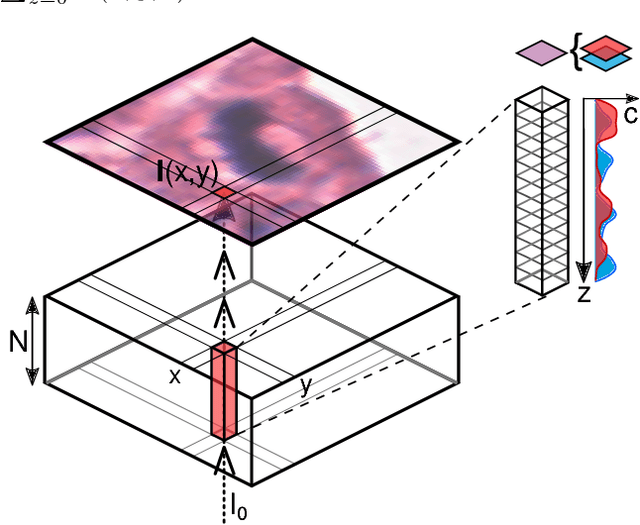
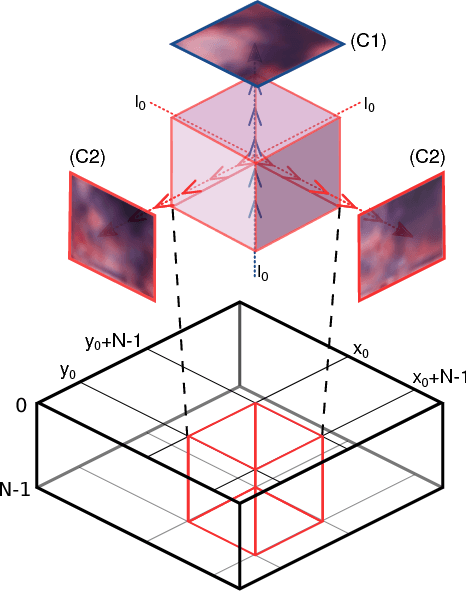
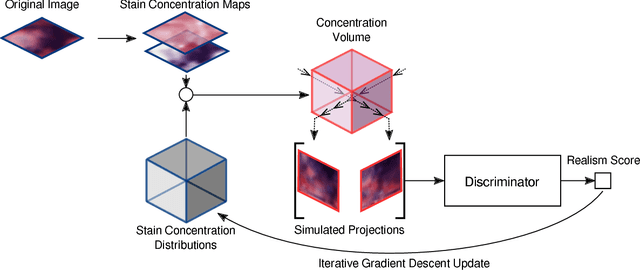

Abstract:Histological images are obtained by transmitting light through a tissue specimen that has been stained in order to produce contrast. This process results in 2D images of the specimen that has a three-dimensional structure. In this paper, we propose a method to infer how the stains are distributed in the direction perpendicular to the surface of the slide for a given 2D image in order to obtain a 3D representation of the tissue. This inference is achieved by decomposition of the staining concentration maps under constraints that ensure realistic decomposition and reconstruction of the original 2D images. Our study shows that it is possible to generate realistic 3D images making this method a potential tool for data augmentation when training deep learning models.
Isointense infant brain MRI segmentation with a dilated convolutional neural network
Aug 09, 2017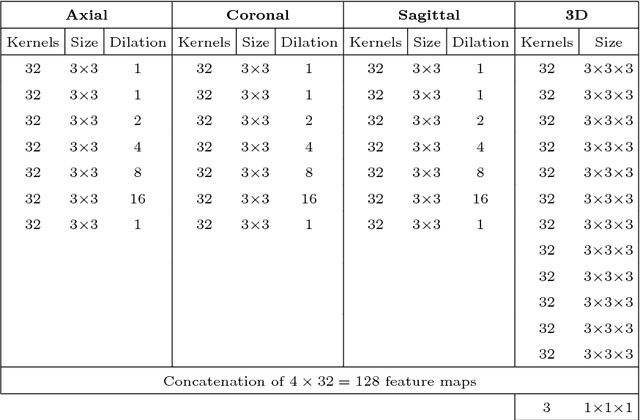


Abstract:Quantitative analysis of brain MRI at the age of 6 months is difficult because of the limited contrast between white matter and gray matter. In this study, we use a dilated triplanar convolutional neural network in combination with a non-dilated 3D convolutional neural network for the segmentation of white matter, gray matter and cerebrospinal fluid in infant brain MR images, as provided by the MICCAI grand challenge on 6-month infant brain MRI segmentation.
Domain-adversarial neural networks to address the appearance variability of histopathology images
Jul 19, 2017



Abstract:Preparing and scanning histopathology slides consists of several steps, each with a multitude of parameters. The parameters can vary between pathology labs and within the same lab over time, resulting in significant variability of the tissue appearance that hampers the generalization of automatic image analysis methods. Typically, this is addressed with ad-hoc approaches such as staining normalization that aim to reduce the appearance variability. In this paper, we propose a systematic solution based on domain-adversarial neural networks. We hypothesize that removing the domain information from the model representation leads to better generalization. We tested our hypothesis for the problem of mitosis detection in breast cancer histopathology images and made a comparative analysis with two other approaches. We show that combining color augmentation with domain-adversarial training is a better alternative than standard approaches to improve the generalization of deep learning methods.
Adversarial training and dilated convolutions for brain MRI segmentation
Jul 11, 2017


Abstract:Convolutional neural networks (CNNs) have been applied to various automatic image segmentation tasks in medical image analysis, including brain MRI segmentation. Generative adversarial networks have recently gained popularity because of their power in generating images that are difficult to distinguish from real images. In this study we use an adversarial training approach to improve CNN-based brain MRI segmentation. To this end, we include an additional loss function that motivates the network to generate segmentations that are difficult to distinguish from manual segmentations. During training, this loss function is optimised together with the conventional average per-voxel cross entropy loss. The results show improved segmentation performance using this adversarial training procedure for segmentation of two different sets of images and using two different network architectures, both visually and in terms of Dice coefficients.
Exploring the similarity of medical imaging classification problems
Jun 12, 2017



Abstract:Supervised learning is ubiquitous in medical image analysis. In this paper we consider the problem of meta-learning -- predicting which methods will perform well in an unseen classification problem, given previous experience with other classification problems. We investigate the first step of such an approach: how to quantify the similarity of different classification problems. We characterize datasets sampled from six classification problems by performance ranks of simple classifiers, and define the similarity by the inverse of Euclidean distance in this meta-feature space. We visualize the similarities in a 2D space, where meaningful clusters start to emerge, and show that the proposed representation can be used to classify datasets according to their origin with 89.3\% accuracy. These findings, together with the observations of recent trends in machine learning, suggest that meta-learning could be a valuable tool for the medical imaging community.
Deep Learning for Multi-Task Medical Image Segmentation in Multiple Modalities
Apr 11, 2017


Abstract:Automatic segmentation of medical images is an important task for many clinical applications. In practice, a wide range of anatomical structures are visualised using different imaging modalities. In this paper, we investigate whether a single convolutional neural network (CNN) can be trained to perform different segmentation tasks. A single CNN is trained to segment six tissues in MR brain images, the pectoral muscle in MR breast images, and the coronary arteries in cardiac CTA. The CNN therefore learns to identify the imaging modality, the visualised anatomical structures, and the tissue classes. For each of the three tasks (brain MRI, breast MRI and cardiac CTA), this combined training procedure resulted in a segmentation performance equivalent to that of a CNN trained specifically for that task, demonstrating the high capacity of CNN architectures. Hence, a single system could be used in clinical practice to automatically perform diverse segmentation tasks without task-specific training.
Automatic segmentation of MR brain images with a convolutional neural network
Apr 11, 2017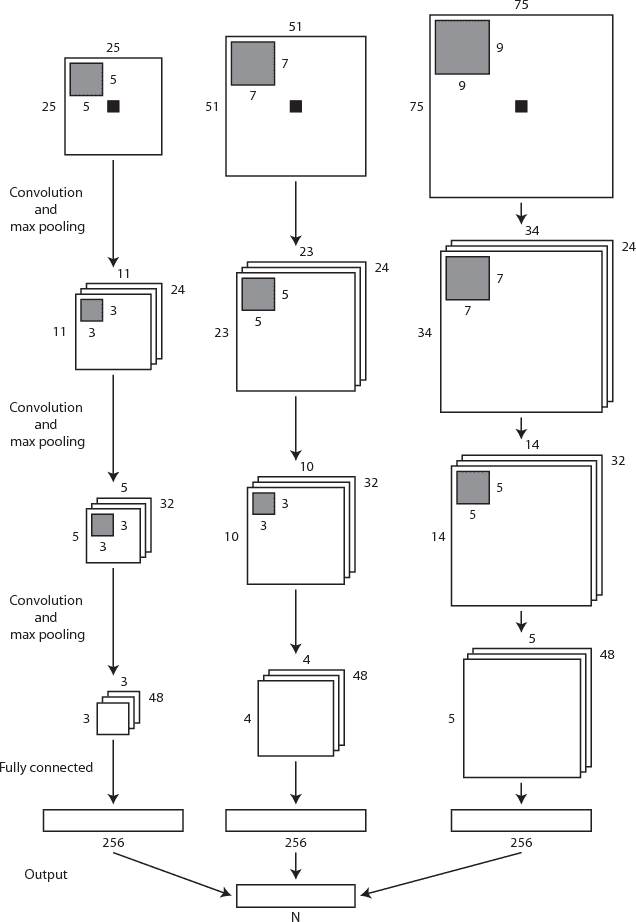

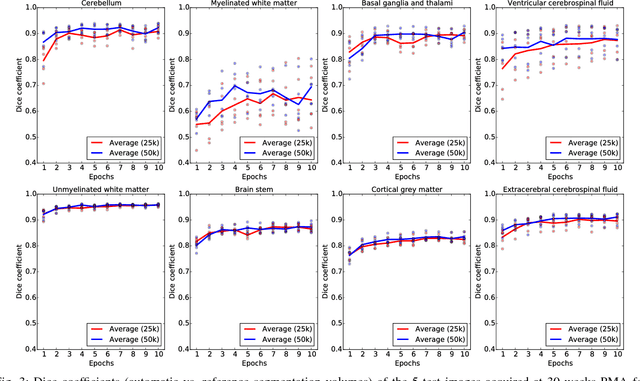

Abstract:Automatic segmentation in MR brain images is important for quantitative analysis in large-scale studies with images acquired at all ages. This paper presents a method for the automatic segmentation of MR brain images into a number of tissue classes using a convolutional neural network. To ensure that the method obtains accurate segmentation details as well as spatial consistency, the network uses multiple patch sizes and multiple convolution kernel sizes to acquire multi-scale information about each voxel. The method is not dependent on explicit features, but learns to recognise the information that is important for the classification based on training data. The method requires a single anatomical MR image only. The segmentation method is applied to five different data sets: coronal T2-weighted images of preterm infants acquired at 30 weeks postmenstrual age (PMA) and 40 weeks PMA, axial T2- weighted images of preterm infants acquired at 40 weeks PMA, axial T1-weighted images of ageing adults acquired at an average age of 70 years, and T1-weighted images of young adults acquired at an average age of 23 years. The method obtained the following average Dice coefficients over all segmented tissue classes for each data set, respectively: 0.87, 0.82, 0.84, 0.86 and 0.91. The results demonstrate that the method obtains accurate segmentations in all five sets, and hence demonstrates its robustness to differences in age and acquisition protocol.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge