Michael Sühling
Neural Image Unfolding: Flattening Sparse Anatomical Structures using Neural Fields
Nov 27, 2024



Abstract:Tomographic imaging reveals internal structures of 3D objects and is crucial for medical diagnoses. Visualizing the morphology and appearance of non-planar sparse anatomical structures that extend over multiple 2D slices in tomographic volumes is inherently difficult but valuable for decision-making and reporting. Hence, various organ-specific unfolding techniques exist to map their densely sampled 3D surfaces to a distortion-minimized 2D representation. However, there is no versatile framework to flatten complex sparse structures including vascular, duct or bone systems. We deploy a neural field to fit the transformation of the anatomy of interest to a 2D overview image. We further propose distortion regularization strategies and combine geometric with intensity-based loss formulations to also display non-annotated and auxiliary targets. In addition to improved versatility, our unfolding technique outperforms mesh-based baselines for sparse structures w.r.t. peak distortion and our regularization scheme yields smoother transformations compared to Jacobian formulations from neural field-based image registration.
CAD-RADS Scoring using Deep Learning and Task-Specific Centerline Labeling
Feb 08, 2022



Abstract:With coronary artery disease (CAD) persisting to be one of the leading causes of death worldwide, interest in supporting physicians with algorithms to speed up and improve diagnosis is high. In clinical practice, the severeness of CAD is often assessed with a coronary CT angiography (CCTA) scan and manually graded with the CAD-Reporting and Data System (CAD-RADS) score. The clinical questions this score assesses are whether patients have CAD or not (rule-out) and whether they have severe CAD or not (hold-out). In this work, we reach new state-of-the-art performance for automatic CAD-RADS scoring. We propose using severity-based label encoding, test time augmentation (TTA) and model ensembling for a task-specific deep learning architecture. Furthermore, we introduce a novel task- and model-specific, heuristic coronary segment labeling, which subdivides coronary trees into consistent parts across patients. It is fast, robust, and easy to implement. We were able to raise the previously reported area under the receiver operating characteristic curve (AUC) from 0.914 to 0.942 in the rule-out and from 0.921 to 0.950 in the hold-out task respectively.
Coronary Plaque Analysis for CT Angiography Clinical Research
Jan 11, 2021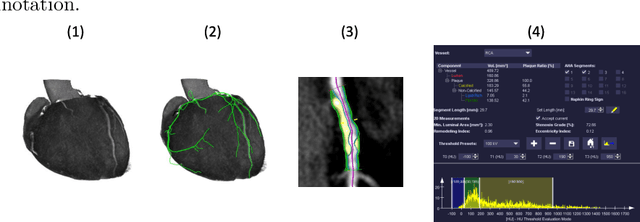
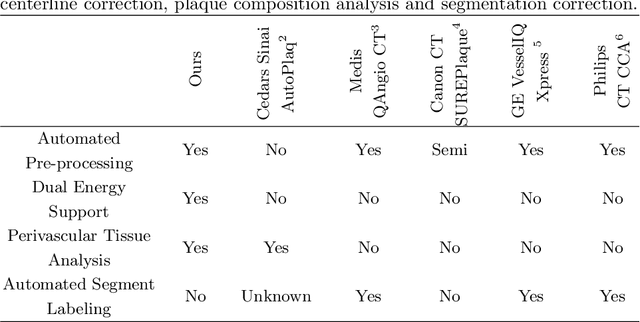
Abstract:The analysis of plaque deposits in the coronary vasculature is an important topic in current clinical research. From a technical side mostly new algorithms for different sub tasks - e.g. centerline extraction or vessel/plaque segmentation - are proposed. However, to enable clinical research with the help of these algorithms, a software solution, which enables manual correction, comprehensive visual feedback and tissue analysis capabilities, is needed. Therefore, we want to present such an integrated software solution. It is able to perform robust automatic centerline extraction and inner and outer vessel wall segmentation, while providing easy to use manual correction tools. Also, it allows for annotation of lesions along the centerlines, which can be further analyzed regarding their tissue composition. Furthermore, it enables research in upcoming technologies and research directions: it does support dual energy CT scans with dedicated plaque analysis and the quantification of the fatty tissue surrounding the vasculature, also in automated set-ups.
Explaining Clinical Decision Support Systems in Medical Imaging using Cycle-Consistent Activation Maximization
Oct 13, 2020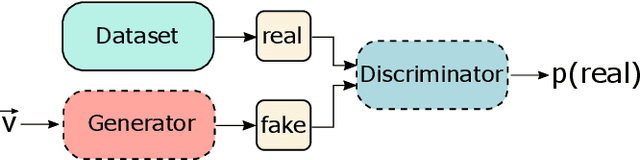
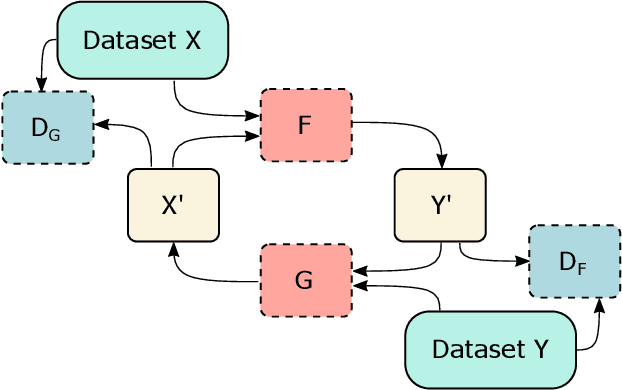
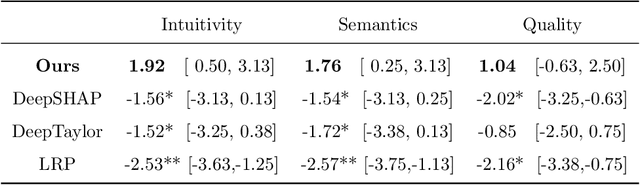
Abstract:Clinical decision support using deep neural networks has become a topic of steadily growing interest. While recent work has repeatedly demonstrated that deep learning offers major advantages for medical image classification over traditional methods, clinicians are often hesitant to adopt the technology because its underlying decision-making process is considered to be intransparent and difficult to comprehend. In recent years, this has been addressed by a variety of approaches that have successfully contributed to providing deeper insight. Most notably, additive feature attribution methods are able to propagate decisions back into the input space by creating a saliency map which allows the practitioner to "see what the network sees." However, the quality of the generated maps can become poor and the images noisy if only limited data is available - a typical scenario in clinical contexts. We propose a novel decision explanation scheme based on CycleGAN activation maximization which generates high-quality visualizations of classifier decisions even in smaller data sets. We conducted a user study in which these visualizations significantly outperformed existing methods on the LIDC dataset for lung lesion malignancy classification. With our approach we make a significant contribution to a better understanding of clinical decision support systems based on deep neural networks and thus aim to foster overall clinical acceptance.
Automatic CAD-RADS Scoring Using Deep Learning
Oct 05, 2020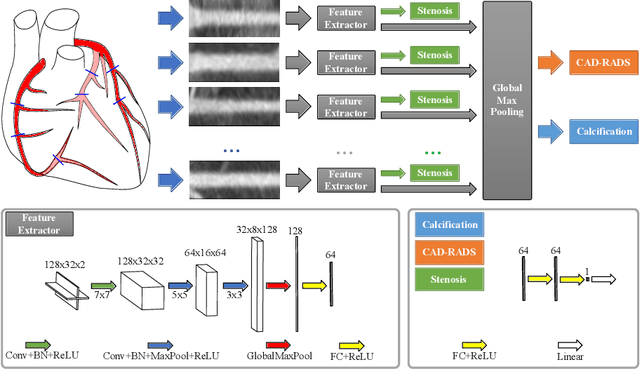

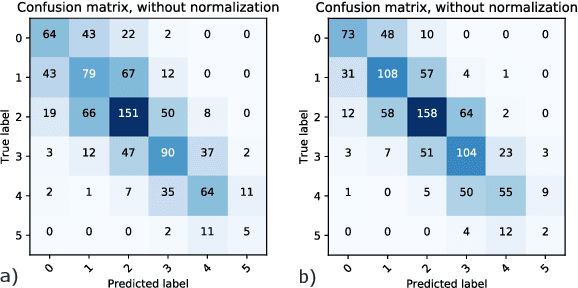
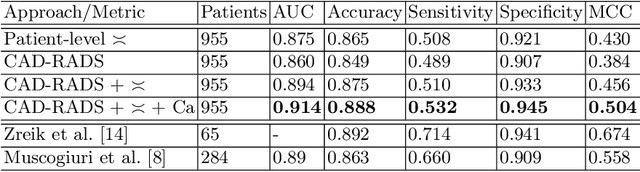
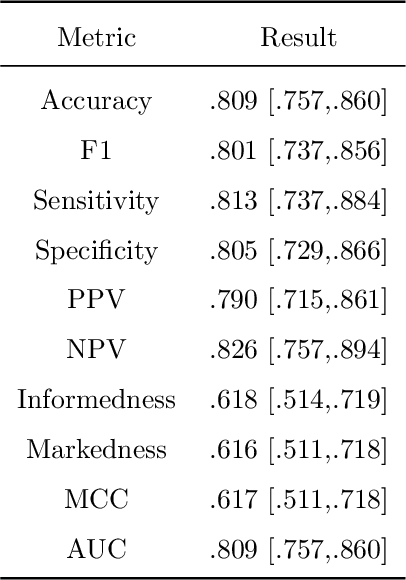
Abstract:Coronary CT angiography (CCTA) has established its role as a non-invasive modality for the diagnosis of coronary artery disease (CAD). The CAD-Reporting and Data System (CAD-RADS) has been developed to standardize communication and aid in decision making based on CCTA findings. The CAD-RADS score is determined by manual assessment of all coronary vessels and the grading of lesions within the coronary artery tree. We propose a bottom-up approach for fully-automated prediction of this score using deep-learning operating on a segment-wise representation of the coronary arteries. The method relies solely on a prior fully-automated centerline extraction and segment labeling and predicts the segment-wise stenosis degree and the overall calcification grade as auxiliary tasks in a multi-task learning setup. We evaluate our approach on a data collection consisting of 2,867 patients. On the task of identifying patients with a CAD-RADS score indicating the need for further invasive investigation our approach reaches an area under curve (AUC) of 0.923 and an AUC of 0.914 for determining whether the patient suffers from CAD. This level of performance enables our approach to be used in a fully-automated screening setup or to assist diagnostic CCTA reading, especially due to its neural architecture design -- which allows comprehensive predictions.
Coronary Artery Plaque Characterization from CCTA Scans using Deep Learning and Radiomics
Dec 13, 2019



Abstract:Assessing coronary artery plaque segments in coronary CT angiography scans is an important task to improve patient management and clinical outcomes, as it can help to decide whether invasive investigation and treatment are necessary. In this work, we present three machine learning approaches capable of performing this task. The first approach is based on radiomics, where a plaque segmentation is used to calculate various shape-, intensity- and texture-based features under different image transformations. A second approach is based on deep learning and relies on centerline extraction as sole prerequisite. In the third approach, we fuse the deep learning approach with radiomic features. On our data the methods reached similar scores as simulated fractional flow reserve (FFR) measurements, which - in contrast to our methods - requires an exact segmentation of the whole coronary tree and often time-consuming manual interaction. In literature, the performance of simulated FFR reaches an AUC between 0.79-0.93 predicting an abnormal invasive FFR that demands revascularization. The radiomics approach achieves an AUC of 0.86, the deep learning approach 0.84 and the combined method 0.88 for predicting the revascularization decision directly. While all three proposed methods can be determined within seconds, the FFR simulation typically takes several minutes. Provided representative training data in sufficient quantities, we believe that the presented methods can be used to create systems for fully automatic non-invasive risk assessment for a variety of adverse cardiac events.
Deep Learning Algorithms for Coronary Artery Plaque Characterisation from CCTA Scans
Dec 13, 2019



Abstract:Analysing coronary artery plaque segments with respect to their functional significance and therefore their influence to patient management in a non-invasive setup is an important subject of current research. In this work we compare and improve three deep learning algorithms for this task: A 3D recurrent convolutional neural network (RCNN), a 2D multi-view ensemble approach based on texture analysis, and a newly proposed 2.5D approach. Current state of the art methods utilising fluid dynamics based fractional flow reserve (FFR) simulation reach an AUC of up to 0.93 for the task of predicting an abnormal invasive FFR value. For the comparable task of predicting revascularisation decision, we are able to improve the performance in terms of AUC of both existing approaches with the proposed modifications, specifically from 0.80 to 0.90 for the 3D-RCNN, and from 0.85 to 0.90 for the multi-view texture-based ensemble. The newly proposed 2.5D approach achieves comparable results with an AUC of 0.90.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge