Matthew Peterson
Final Report on MITRE Evaluations for the DARPA Big Mechanism Program
Nov 08, 2022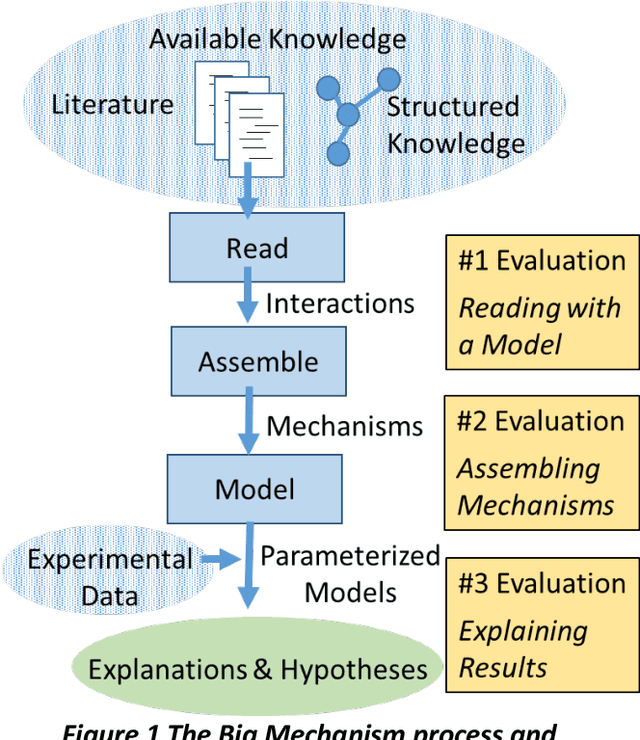
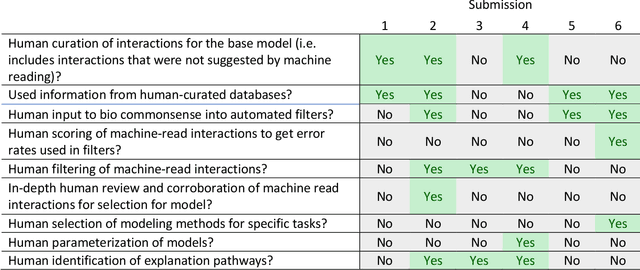
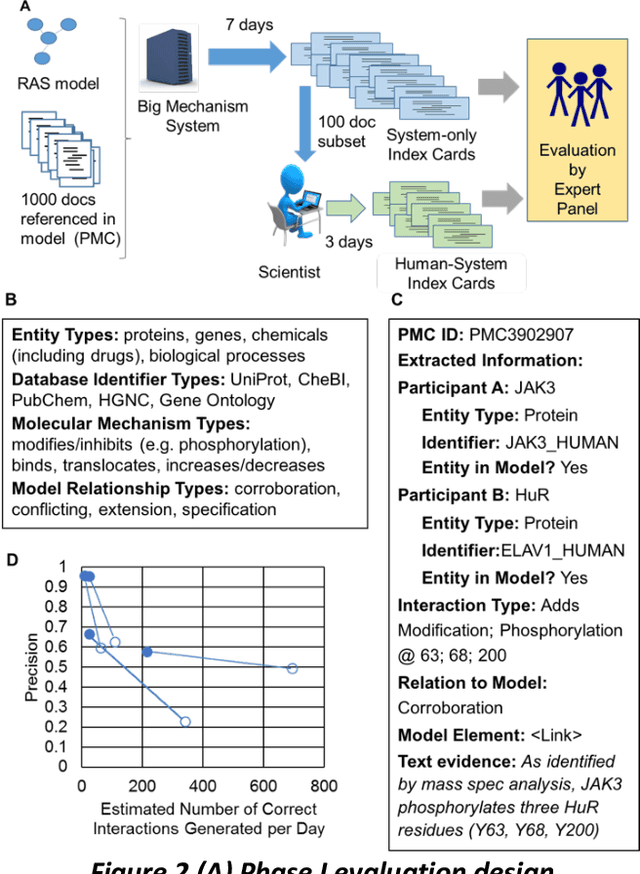
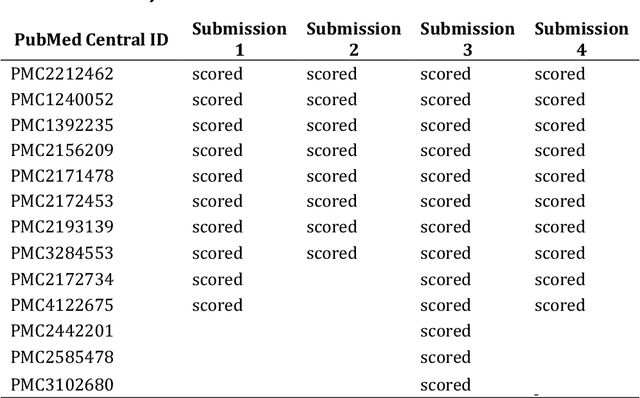
Abstract:This report presents the evaluation approach developed for the DARPA Big Mechanism program, which aimed at developing computer systems that will read research papers, integrate the information into a computer model of cancer mechanisms, and frame new hypotheses. We employed an iterative, incremental approach to the evaluation of the three phases of the program. In Phase I, we evaluated the ability of system and human teams ability to read-with-a-model to capture mechanistic information from the biomedical literature, integrated with information from expert curated biological databases. In Phase II we evaluated the ability of systems to assemble fragments of information into a mechanistic model. The Phase III evaluation focused on the ability of systems to provide explanations of experimental observations based on models assembled (largely automatically) by the Big Mechanism process. The evaluation for each phase built on earlier evaluations and guided developers towards creating capabilities for the new phase. The report describes our approach, including innovations such as a reference set (a curated data set limited to major findings of each paper) to assess the accuracy of systems in extracting mechanistic findings in the absence of a gold standard, and a method to evaluate model-based explanations of experimental data. Results of the evaluation and supporting materials are included in the appendices.
The state of the art in kidney and kidney tumor segmentation in contrast-enhanced CT imaging: Results of the KiTS19 Challenge
Dec 02, 2019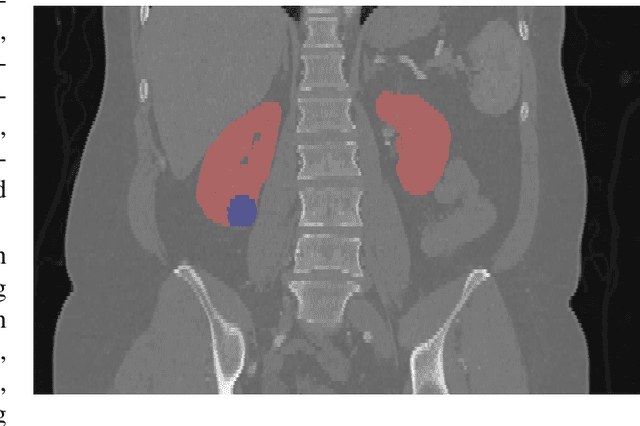
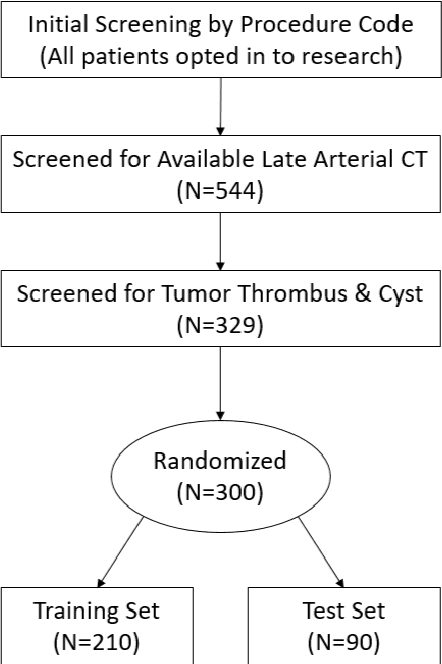
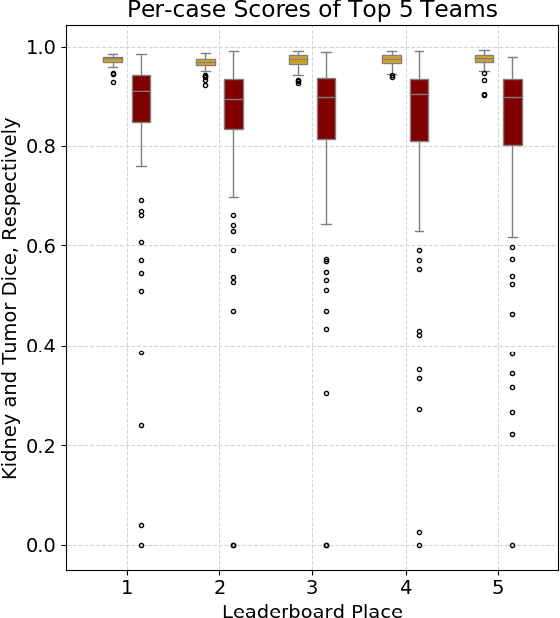
Abstract:There is a large body of literature linking anatomic and geometric characteristics of kidney tumors to perioperative and oncologic outcomes. Semantic segmentation of these tumors and their host kidneys is a promising tool for quantitatively characterizing these lesions, but its adoption is limited due to the manual effort required to produce high-quality 3D segmentations of these structures. Recently, methods based on deep learning have shown excellent results in automatic 3D segmentation, but they require large datasets for training, and there remains little consensus on which methods perform best. The 2019 Kidney and Kidney Tumor Segmentation challenge (KiTS19) was a competition held in conjunction with the 2019 International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI) which sought to address these issues and stimulate progress on this automatic segmentation problem. A training set of 210 cross sectional CT images with kidney tumors was publicly released with corresponding semantic segmentation masks. 106 teams from five continents used this data to develop automated systems to predict the true segmentation masks on a test set of 90 CT images for which the corresponding ground truth segmentations were kept private. These predictions were scored and ranked according to their average So rensen-Dice coefficient between the kidney and tumor across all 90 cases. The winning team achieved a Dice of 0.974 for kidney and 0.851 for tumor, approaching the inter-annotator performance on kidney (0.983) but falling short on tumor (0.923). This challenge has now entered an "open leaderboard" phase where it serves as a challenging benchmark in 3D semantic segmentation.
Counterfactual Vision-and-Language Navigation via Adversarial Path Sampling
Nov 17, 2019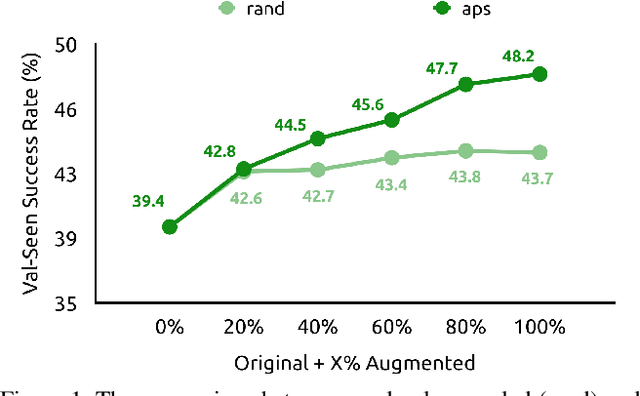
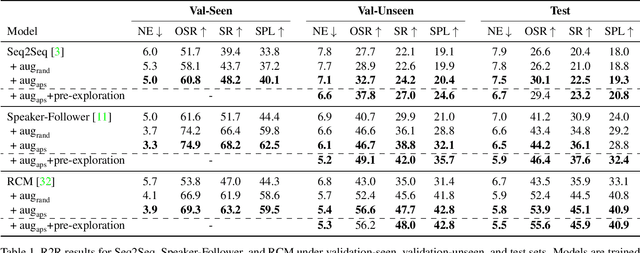
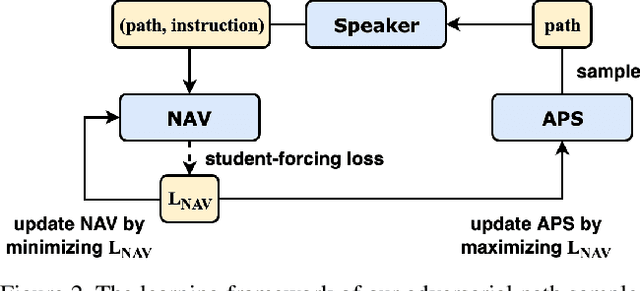
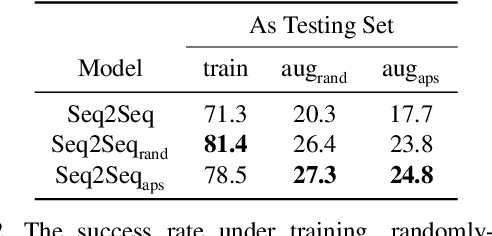
Abstract:Vision-and-Language Navigation (VLN) is a task where agents must decide how to move through a 3D environment to reach a goal by grounding natural language instructions to the visual surroundings. One of the problems of the VLN task is data scarcity since it is difficult to collect enough navigation paths with human-annotated instructions for interactive environments. In this paper, we explore the use of counterfactual thinking as a human-inspired data augmentation method that results in robust models. Counterfactual thinking is a concept that describes the human propensity to create possible alternatives to life events that have already occurred. We propose an adversarial-driven counterfactual reasoning model that can consider effective conditions instead of low-quality augmented data. In particular, we present a model-agnostic adversarial path sampler (APS) that learns to sample challenging paths that force the navigator to improve based on the navigation performance. APS also serves to do pre-exploration of unseen environments to strengthen the model's ability to generalize. We evaluate the influence of APS on the performance of different VLN baseline models using the room-to-room dataset (R2R). The results show that the adversarial training process with our proposed APS benefits VLN models under both seen and unseen environments. And the pre-exploration process can further gain additional improvements under unseen environments.
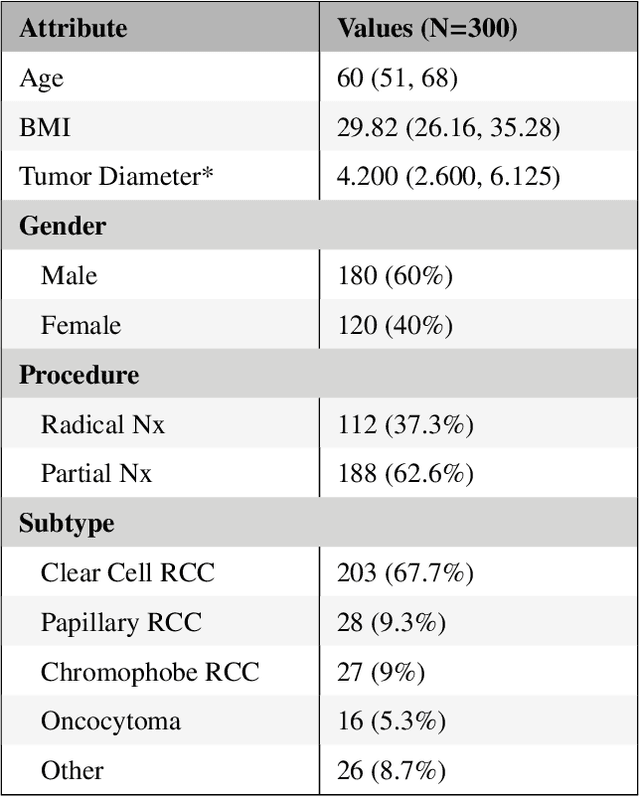
The KiTS19 Challenge Data: 300 Kidney Tumor Cases with Clinical Context, CT Semantic Segmentations, and Surgical Outcomes
Mar 31, 2019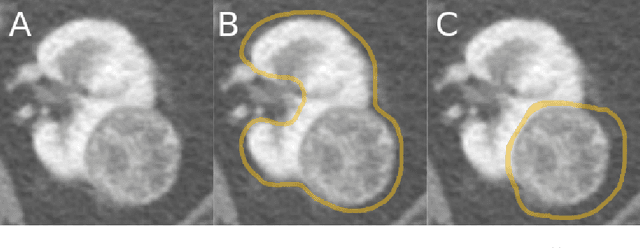
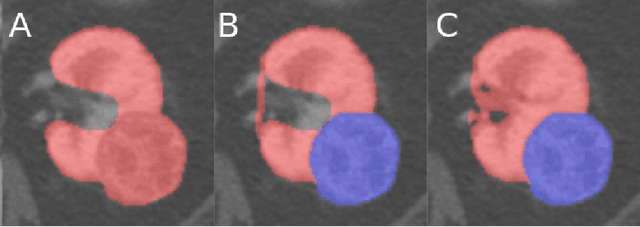
Abstract:The morphometry of a kidney tumor revealed by contrast-enhanced Computed Tomography (CT) imaging is an important factor in clinical decision making surrounding the lesion's diagnosis and treatment. Quantitative study of the relationship between kidney tumor morphology and clinical outcomes is difficult due to data scarcity and the laborious nature of manually quantifying imaging predictors. Automatic semantic segmentation of kidneys and kidney tumors is a promising tool towards automatically quantifying a wide array of morphometric features, but no sizeable annotated dataset is currently available to train models for this task. We present the KiTS19 challenge dataset: A collection of multi-phase CT imaging, segmentation masks, and comprehensive clinical outcomes for 300 patients who underwent nephrectomy for kidney tumors at our center between 2010 and 2018. 210 (70%) of these patients were selected at random as the training set for the 2019 MICCAI KiTS Kidney Tumor Segmentation Challenge and have been released publicly. With the presence of clinical context and surgical outcomes, this data can serve not only for benchmarking semantic segmentation models, but also for developing and studying biomarkers which make use of the imaging and semantic segmentation masks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge