Martin Weigert
Sequence models for continuous cell cycle stage prediction from brightfield images
Feb 04, 2025Abstract:Understanding cell cycle dynamics is crucial for studying biological processes such as growth, development and disease progression. While fluorescent protein reporters like the Fucci system allow live monitoring of cell cycle phases, they require genetic engineering and occupy additional fluorescence channels, limiting broader applicability in complex experiments. In this study, we conduct a comprehensive evaluation of deep learning methods for predicting continuous Fucci signals using non-fluorescence brightfield imaging, a widely available label-free modality. To that end, we generated a large dataset of 1.3 M images of dividing RPE1 cells with full cell cycle trajectories to quantitatively compare the predictive performance of distinct model categories including single time-frame models, causal state space models and bidirectional transformer models. We show that both causal and transformer-based models significantly outperform single- and fixed frame approaches, enabling the prediction of visually imperceptible transitions like G1/S within 1h resolution. Our findings underscore the importance of sequence models for accurate predictions of cell cycle dynamics and highlight their potential for label-free imaging.
Trackastra: Transformer-based cell tracking for live-cell microscopy
May 24, 2024Abstract:Cell tracking is an omnipresent image analysis task in live-cell microscopy. It is similar to multiple object tracking (MOT), however, each frame contains hundreds of similar-looking objects that can divide, making it a challenging problem. Current state-of-the-art approaches follow the tracking-by-detection paradigm, i.e. first all cells are detected per frame and successively linked in a second step to form biologically consistent cell tracks. Linking is commonly solved via discrete optimization methods, which require manual tuning of hyperparameters for each dataset and are therefore cumbersome to use in practice. Here we propose Trackastra, a general purpose cell tracking approach that uses a simple transformer architecture to directly learn pairwise associations of cells within a temporal window from annotated data. Importantly, unlike existing transformer-based MOT pipelines, our learning architecture also accounts for dividing objects such as cells and allows for accurate tracking even with simple greedy linking, thus making strides towards removing the requirement for a complex linking step. The proposed architecture operates on the full spatio-temporal context of detections within a time window by avoiding the computational burden of processing dense images. We show that our tracking approach performs on par with or better than highly tuned state-of-the-art cell tracking algorithms for various biological datasets, such as bacteria, cell cultures and fluorescent particles. We provide code at https://github.com/weigertlab/trackastra.
Self-supervised dense representation learning for live-cell microscopy with time arrow prediction
May 09, 2023Abstract:State-of-the-art object detection and segmentation methods for microscopy images rely on supervised machine learning, which requires laborious manual annotation of training data. Here we present a self-supervised method based on time arrow prediction pre-training that learns dense image representations from raw, unlabeled live-cell microscopy videos. Our method builds upon the task of predicting the correct order of time-flipped image regions via a single-image feature extractor and a subsequent time arrow prediction head. We show that the resulting dense representations capture inherently time-asymmetric biological processes such as cell divisions on a pixel-level. We furthermore demonstrate the utility of these representations on several live-cell microscopy datasets for detection and segmentation of dividing cells, as well as for cell state classification. Our method outperforms supervised methods, particularly when only limited ground truth annotations are available as is commonly the case in practice. We provide code at https://github.com/weigertlab/tarrow.
CoNIC Challenge: Pushing the Frontiers of Nuclear Detection, Segmentation, Classification and Counting
Mar 14, 2023



Abstract:Nuclear detection, segmentation and morphometric profiling are essential in helping us further understand the relationship between histology and patient outcome. To drive innovation in this area, we setup a community-wide challenge using the largest available dataset of its kind to assess nuclear segmentation and cellular composition. Our challenge, named CoNIC, stimulated the development of reproducible algorithms for cellular recognition with real-time result inspection on public leaderboards. We conducted an extensive post-challenge analysis based on the top-performing models using 1,658 whole-slide images of colon tissue. With around 700 million detected nuclei per model, associated features were used for dysplasia grading and survival analysis, where we demonstrated that the challenge's improvement over the previous state-of-the-art led to significant boosts in downstream performance. Our findings also suggest that eosinophils and neutrophils play an important role in the tumour microevironment. We release challenge models and WSI-level results to foster the development of further methods for biomarker discovery.
Organelle-specific segmentation, spatial analysis, and visualization of volume electron microscopy datasets
Mar 07, 2023Abstract:Volume electron microscopy is the method of choice for the in-situ interrogation of cellular ultrastructure at the nanometer scale. Recent technical advances have led to a rapid increase in large raw image datasets that require computational strategies for segmentation and spatial analysis. In this protocol, we describe a practical and annotation-efficient pipeline for organelle-specific segmentation, spatial analysis, and visualization of large volume electron microscopy datasets using freely available, user-friendly software tools that can be run on a single standard workstation. We specifically target researchers in the life sciences with limited computational expertise, who face the following tasks within their volume electron microscopy projects: i) How to generate 3D segmentation labels for different types of cell organelles while minimizing manual annotation efforts, ii) how to analyze the spatial interactions between organelle instances, and iii) how to best visualize the 3D segmentation results. To meet these demands we give detailed guidelines for choosing the most efficient segmentation tools for the specific cell organelle. We furthermore provide easily executable components for spatial analysis and 3D rendering and bridge compatibility issues between freely available open-source tools, such that others can replicate our full pipeline starting from a raw dataset up to the final plots and rendered images. We believe that our detailed description can serve as a valuable reference for similar projects requiring special strategies for single- or multiple organelle analysis which can be achieved with computational resources commonly available to single-user setups.
Nuclei instance segmentation and classification in histopathology images with StarDist
Mar 24, 2022

Abstract:Instance segmentation and classification of nuclei is an important task in computational pathology. We show that StarDist, a deep learning based nuclei segmentation method originally developed for fluorescence microscopy, can be extended and successfully applied to histopathology images. This is substantiated by conducting experiments on the Lizard dataset, and through entering the Colon Nuclei Identification and Counting (CoNIC) challenge 2022. At the end of the preliminary test phase of CoNIC, our approach ranked first on the leaderboard for the segmentation and classification task.
Fast and accurate aberration estimation from 3D bead images using convolutional neural networks
Jun 02, 2020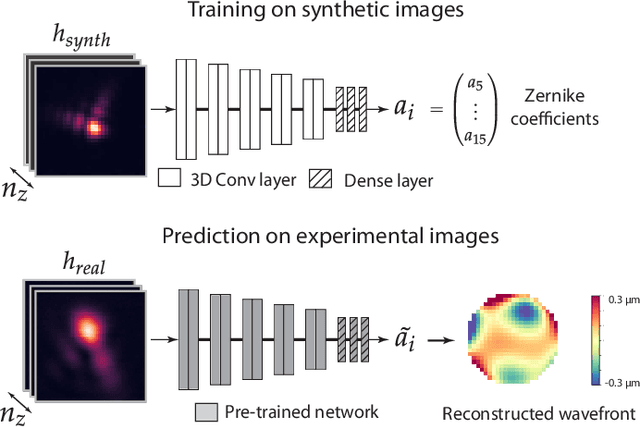

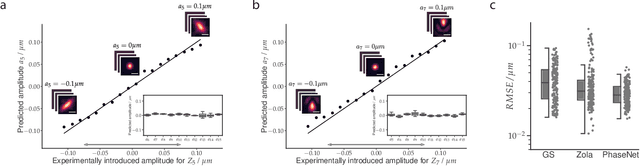
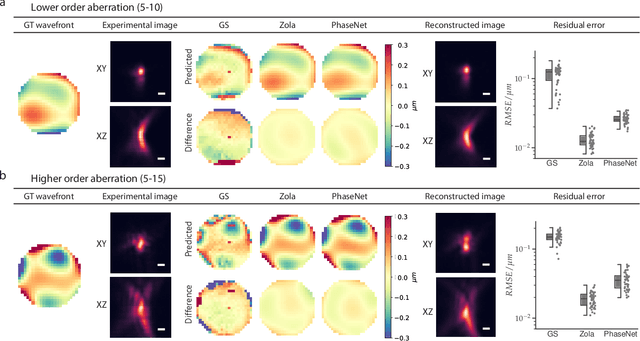
Abstract:Estimating optical aberrations from volumetric intensity images is a key step in sensorless adaptive optics for microscopy. Here we describe a method (PHASENET) for fast and accurate aberration measurement from experimentally acquired 3D bead images using convolutional neural networks. Importantly, we show that networks trained only on synthetically generated data can successfully predict aberrations from experimental images. We demonstrate our approach on two data sets acquired with different microscopy modalities and find that PHASENET yields results better than or comparable to classical methods while being orders of magnitude faster. We furthermore show that the number of focal planes required for satisfactory prediction is related to different symmetry groups of Zernike modes. PHASENET is freely available as open-source software in Python.
An interpretable automated detection system for FISH-based HER2 oncogene amplification testing in histo-pathological routine images of breast and gastric cancer diagnostics
May 25, 2020
Abstract:Histo-pathological diagnostics are an inherent part of the everyday work but are particularly laborious and associated with time-consuming manual analysis of image data. In order to cope with the increasing diagnostic case numbers due to the current growth and demographic change of the global population and the progress in personalized medicine, pathologists ask for assistance. Profiting from digital pathology and the use of artificial intelligence, individual solutions can be offered (e.g. detect labeled cancer tissue sections). The testing of the human epidermal growth factor receptor 2 (HER2) oncogene amplification status via fluorescence in situ hybridization (FISH) is recommended for breast and gastric cancer diagnostics and is regularly performed at clinics. Here, we develop an interpretable, deep learning (DL)-based pipeline which automates the evaluation of FISH images with respect to HER2 gene amplification testing. It mimics the pathological assessment and relies on the detection and localization of interphase nuclei based on instance segmentation networks. Furthermore, it localizes and classifies fluorescence signals within each nucleus with the help of image classification and object detection convolutional neural networks (CNNs). Finally, the pipeline classifies the whole image regarding its HER2 amplification status. The visualization of pixels on which the networks' decision occurs, complements an essential part to enable interpretability by pathologists.
Star-convex Polyhedra for 3D Object Detection and Segmentation in Microscopy
Aug 09, 2019


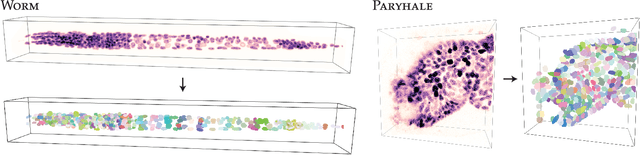
Abstract:Accurate detection and segmentation of cell nuclei in volumetric (3D) fluorescence microscopy datasets is an important step in many biomedical research projects. Although many automated methods for these tasks exist, they often struggle for images with low signal-to-noise ratios and/or dense packing of nuclei. It was recently shown for 2D microscopy images that these issues can be alleviated by training a neural network to directly predict a suitable shape representation (star-convex polygon) for cell nuclei. In this paper, we adopt and extend this approach to 3D volumes by using star-convex polyhedra to represent cell nuclei and similar shapes. To that end, we overcome the challenges of 1) finding parameter-efficient star-convex polyhedra representations that can faithfully describe cell nuclei shapes, 2) adapting to anisotropic voxel sizes often found in fluorescence microscopy datasets, and 3) efficiently computing intersections between pairs of star-convex polyhedra (required for non-maximum suppression). Although our approach is quite general, since star-convex polyhedra subsume common shapes like bounding boxes and spheres as special cases, our focus is on accurate detection and segmentation of cell nuclei. That that end, we demonstrate on two challenging datasets that our approach (StarDist-3D) leads to superior results when compared to classical and deep-learning based methods.
Cell Detection with Star-convex Polygons
Jun 09, 2018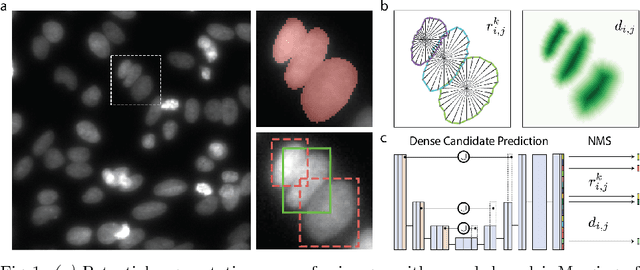

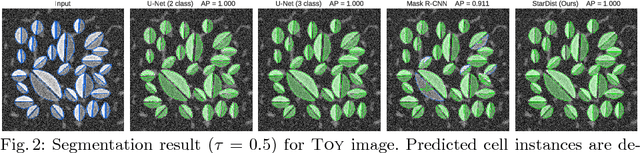
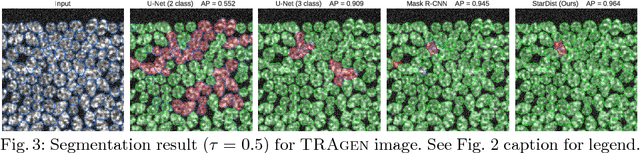
Abstract:Automatic detection and segmentation of cells and nuclei in microscopy images is important for many biological applications. Recent successful learning-based approaches include per-pixel cell segmentation with subsequent pixel grouping, or localization of bounding boxes with subsequent shape refinement. In situations of crowded cells, these can be prone to segmentation errors, such as falsely merging bordering cells or suppressing valid cell instances due to the poor approximation with bounding boxes. To overcome these issues, we propose to localize cell nuclei via star-convex polygons, which are a much better shape representation as compared to bounding boxes and thus do not need shape refinement. To that end, we train a convolutional neural network that predicts for every pixel a polygon for the cell instance at that position. We demonstrate the merits of our approach on two synthetic datasets and one challenging dataset of diverse fluorescence microscopy images.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge