Na Ji
Coordinate-based neural representations for computational adaptive optics in widefield microscopy
Jul 07, 2023Abstract:Widefield microscopy is widely used for non-invasive imaging of biological structures at subcellular resolution. When applied to complex specimen, its image quality is degraded by sample-induced optical aberration. Adaptive optics can correct wavefront distortion and restore diffraction-limited resolution but require wavefront sensing and corrective devices, increasing system complexity and cost. Here, we describe a self-supervised machine learning algorithm, CoCoA, that performs joint wavefront estimation and three-dimensional structural information extraction from a single input 3D image stack without the need for external training dataset. We implemented CoCoA for widefield imaging of mouse brain tissues and validated its performance with direct-wavefront-sensing-based adaptive optics. Importantly, we systematically explored and quantitatively characterized the limiting factors of CoCoA's performance. Using CoCoA, we demonstrated the first in vivo widefield mouse brain imaging using machine-learning-based adaptive optics. Incorporating coordinate-based neural representations and a forward physics model, the self-supervised scheme of CoCoA should be applicable to microscopy modalities in general.
Fast and accurate aberration estimation from 3D bead images using convolutional neural networks
Jun 02, 2020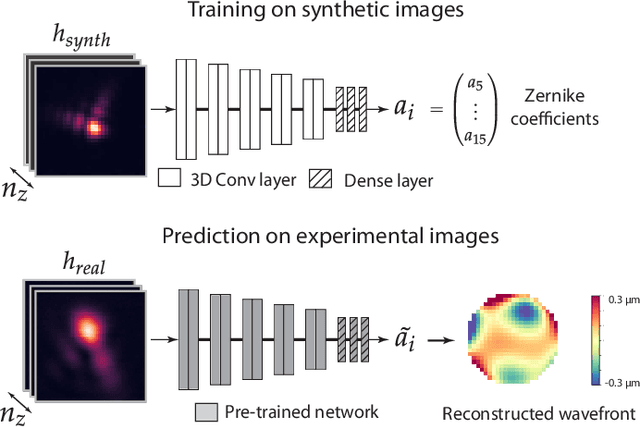

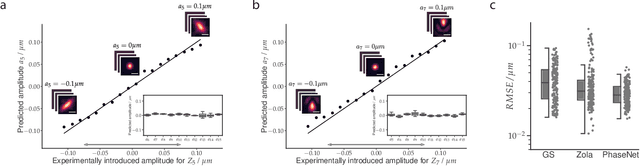
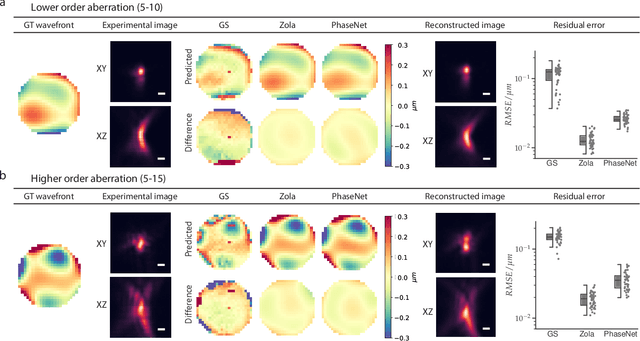
Abstract:Estimating optical aberrations from volumetric intensity images is a key step in sensorless adaptive optics for microscopy. Here we describe a method (PHASENET) for fast and accurate aberration measurement from experimentally acquired 3D bead images using convolutional neural networks. Importantly, we show that networks trained only on synthetically generated data can successfully predict aberrations from experimental images. We demonstrate our approach on two data sets acquired with different microscopy modalities and find that PHASENET yields results better than or comparable to classical methods while being orders of magnitude faster. We furthermore show that the number of focal planes required for satisfactory prediction is related to different symmetry groups of Zernike modes. PHASENET is freely available as open-source software in Python.
Penalized matrix decomposition for denoising, compression, and improved demixing of functional imaging data
Jul 17, 2018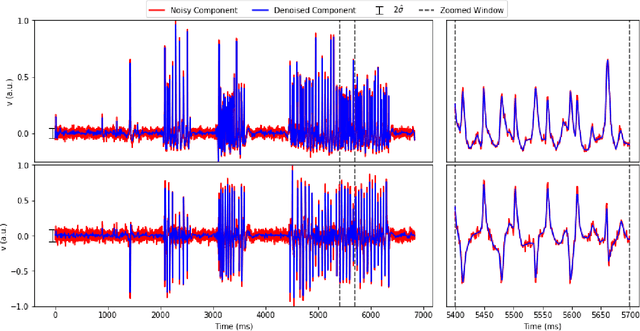
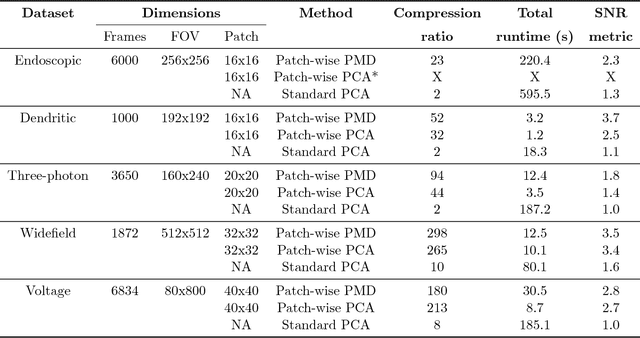
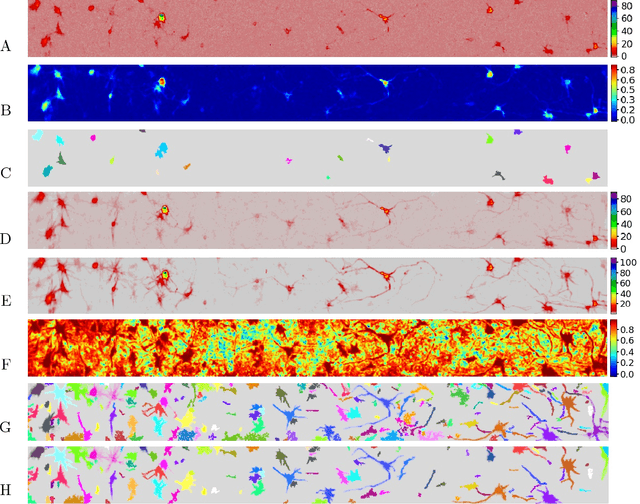
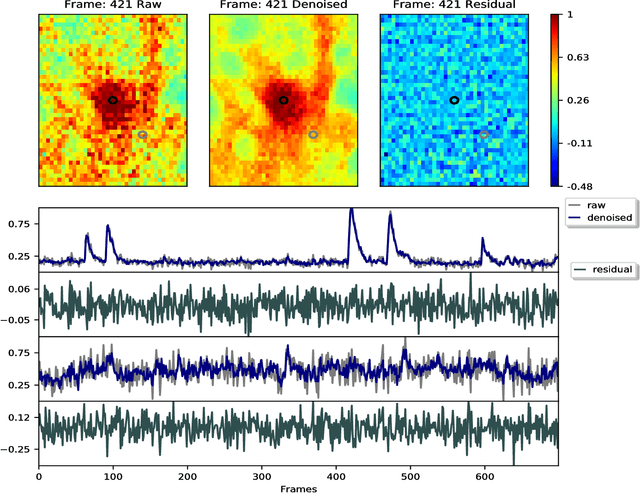
Abstract:Calcium imaging has revolutionized systems neuroscience, providing the ability to image large neural populations with single-cell resolution. The resulting datasets are quite large, which has presented a barrier to routine open sharing of this data, slowing progress in reproducible research. State of the art methods for analyzing this data are based on non-negative matrix factorization (NMF); these approaches solve a non-convex optimization problem, and are effective when good initializations are available, but can break down in low-SNR settings where common initialization approaches fail. Here we introduce an approach to compressing and denoising functional imaging data. The method is based on a spatially-localized penalized matrix decomposition (PMD) of the data to separate (low-dimensional) signal from (temporally-uncorrelated) noise. This approach can be applied in parallel on local spatial patches and is therefore highly scalable, does not impose non-negativity constraints or require stringent identifiability assumptions (leading to significantly more robust results compared to NMF), and estimates all parameters directly from the data, so no hand-tuning is required. We have applied the method to a wide range of functional imaging data (including one-photon, two-photon, three-photon, widefield, somatic, axonal, dendritic, calcium, and voltage imaging datasets): in all cases, we observe ~2-4x increases in SNR and compression rates of 20-300x with minimal visible loss of signal, with no adjustment of hyperparameters; this in turn facilitates the process of demixing the observed activity into contributions from individual neurons. We focus on two challenging applications: dendritic calcium imaging data and voltage imaging data in the context of optogenetic stimulation. In both cases, we show that our new approach leads to faster and much more robust extraction of activity from the data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge