Maria Ines Meyer
ISLES 2022: A multi-center magnetic resonance imaging stroke lesion segmentation dataset
Jun 14, 2022
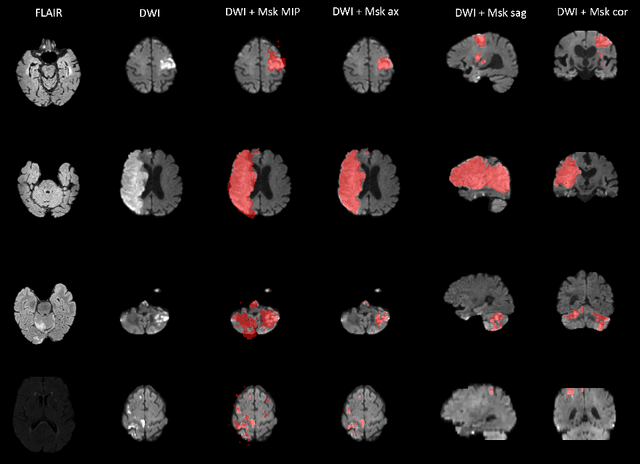

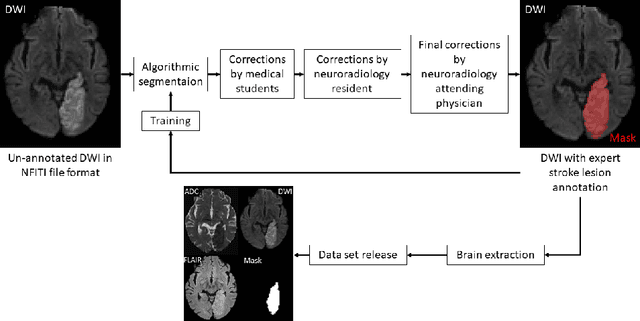
Abstract:Magnetic resonance imaging (MRI) is a central modality for stroke imaging. It is used upon patient admission to make treatment decisions such as selecting patients for intravenous thrombolysis or endovascular therapy. MRI is later used in the duration of hospital stay to predict outcome by visualizing infarct core size and location. Furthermore, it may be used to characterize stroke etiology, e.g. differentiation between (cardio)-embolic and non-embolic stroke. Computer based automated medical image processing is increasingly finding its way into clinical routine. Previous iterations of the Ischemic Stroke Lesion Segmentation (ISLES) challenge have aided in the generation of identifying benchmark methods for acute and sub-acute ischemic stroke lesion segmentation. Here we introduce an expert-annotated, multicenter MRI dataset for segmentation of acute to subacute stroke lesions. This dataset comprises 400 multi-vendor MRI cases with high variability in stroke lesion size, quantity and location. It is split into a training dataset of n=250 and a test dataset of n=150. All training data will be made publicly available. The test dataset will be used for model validation only and will not be released to the public. This dataset serves as the foundation of the ISLES 2022 challenge with the goal of finding algorithmic methods to enable the development and benchmarking of robust and accurate segmentation algorithms for ischemic stroke.
An augmentation strategy to mimic multi-scanner variability in MRI
Mar 23, 2021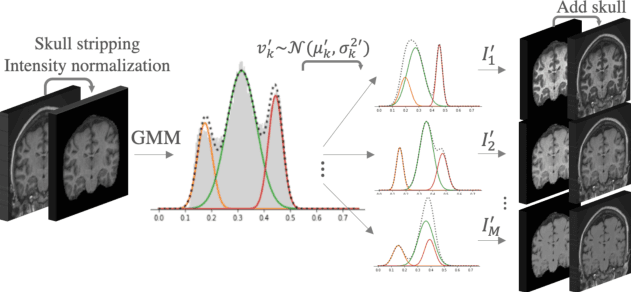
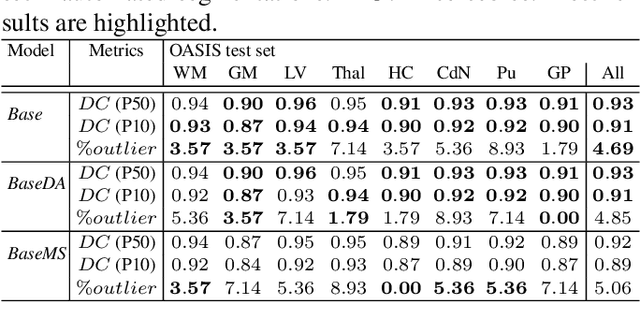
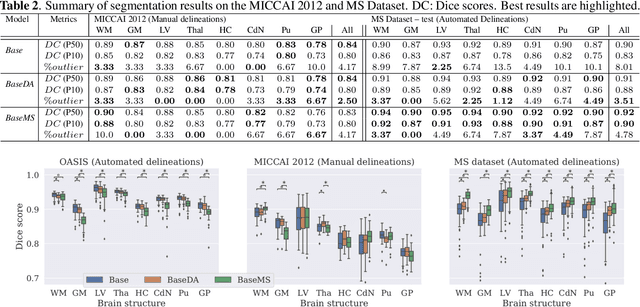
Abstract:Most publicly available brain MRI datasets are very homogeneous in terms of scanner and protocols, and it is difficult for models that learn from such data to generalize to multi-center and multi-scanner data. We propose a novel data augmentation approach with the aim of approximating the variability in terms of intensities and contrasts present in real world clinical data. We use a Gaussian Mixture Model based approach to change tissue intensities individually, producing new contrasts while preserving anatomical information. We train a deep learning model on a single scanner dataset and evaluate it on a multi-center and multi-scanner dataset. The proposed approach improves the generalization capability of the model to other scanners not present in the training data.
Improved inter-scanner MS lesion segmentation by adversarial training on longitudinal data
Feb 03, 2020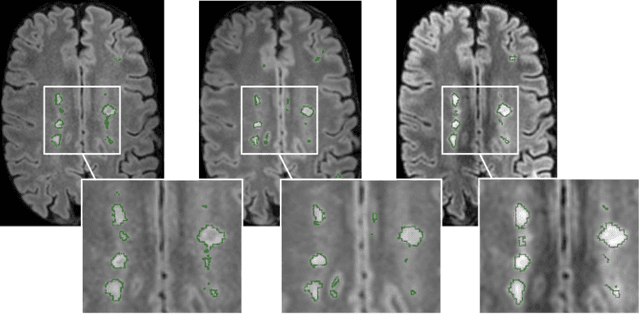
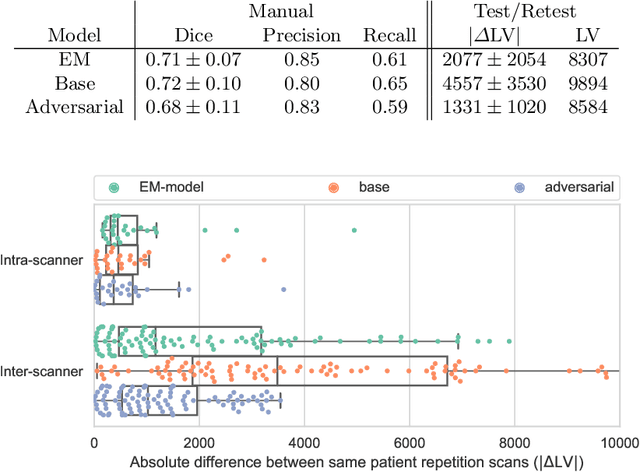
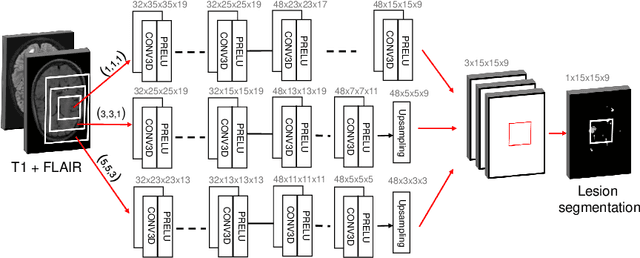
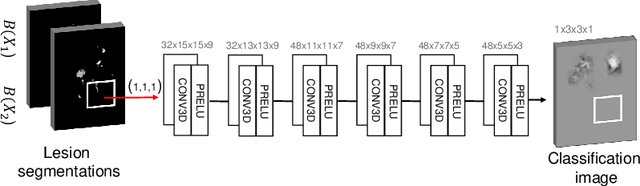
Abstract:The evaluation of white matter lesion progression is an important biomarker in the follow-up of MS patients and plays a crucial role when deciding the course of treatment. Current automated lesion segmentation algorithms are susceptible to variability in image characteristics related to MRI scanner or protocol differences. We propose a model that improves the consistency of MS lesion segmentations in inter-scanner studies. First, we train a CNN base model to approximate the performance of icobrain, an FDA-approved clinically available lesion segmentation software. A discriminator model is then trained to predict if two lesion segmentations are based on scans acquired using the same scanner type or not, achieving a 78% accuracy in this task. Finally, the base model and the discriminator are trained adversarially on multi-scanner longitudinal data to improve the inter-scanner consistency of the base model. The performance of the models is evaluated on an unseen dataset containing manual delineations. The inter-scanner variability is evaluated on test-retest data, where the adversarial network produces improved results over the base model and the FDA-approved solution.
Relevance Vector Machines for harmonization of MRI brain volumes using image descriptors
Nov 08, 2019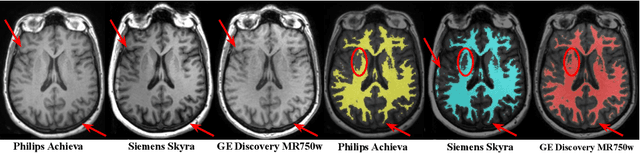
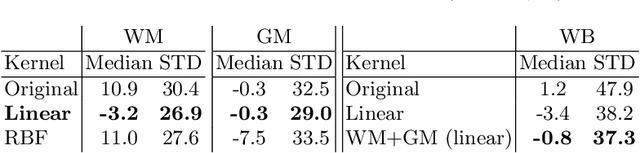
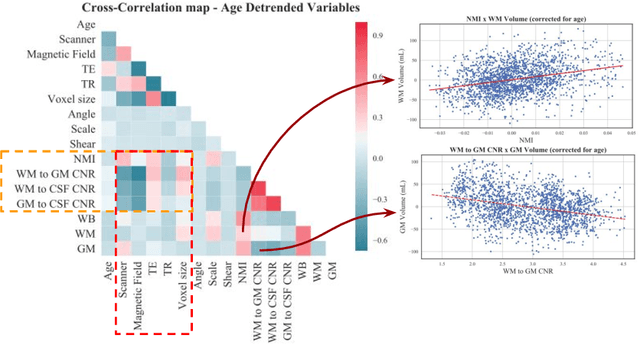
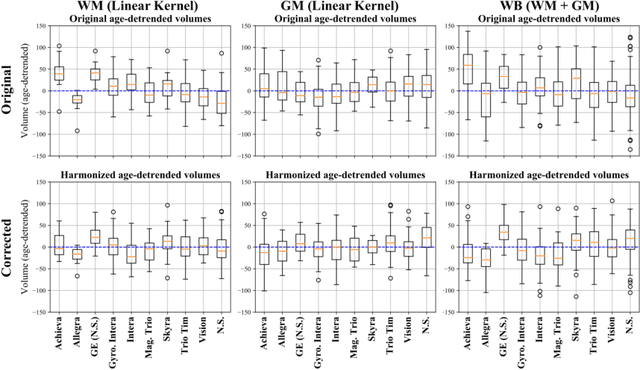
Abstract:With the increased need for multi-center magnetic resonance imaging studies, problems arise related to differences in hardware and software between centers. Namely, current algorithms for brain volume quantification are unreliable for the longitudinal assessment of volume changes in this type of setting. Currently most methods attempt to decrease this issue by regressing the scanner- and/or center-effects from the original data. In this work, we explore a novel approach to harmonize brain volume measurements by using only image descriptors. First, we explore the relationships between volumes and image descriptors. Then, we train a Relevance Vector Machine (RVM) model over a large multi-site dataset of healthy subjects to perform volume harmonization. Finally, we validate the method over two different datasets: i) a subset of unseen healthy controls; and ii) a test-retest dataset of multiple sclerosis (MS) patients. The method decreases scanner and center variability while preserving measurements that did not require correction in MS patient data. We show that image descriptors can be used as input to a machine learning algorithm to improve the reliability of longitudinal volumetric studies.
* 9 pages, 4 figures. Presented at the International Workshop on Machine Learning in Clinical Neuroimaging (MLCN) 2019
Data-Driven Color Augmentation Techniques for Deep Skin Image Analysis
Mar 10, 2017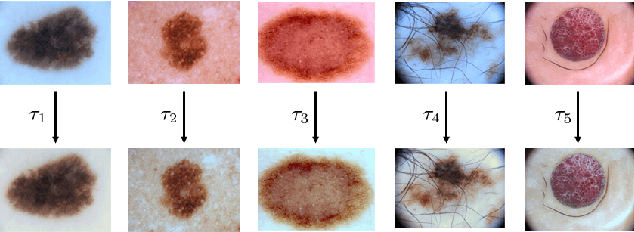
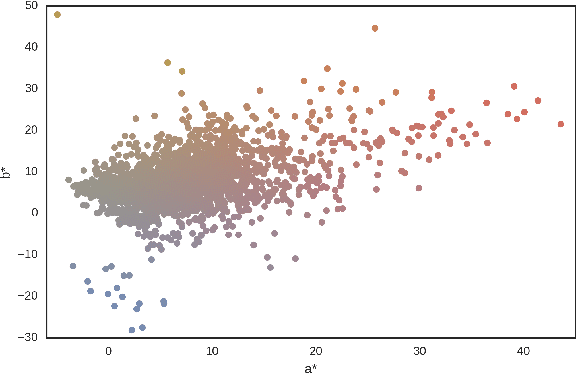
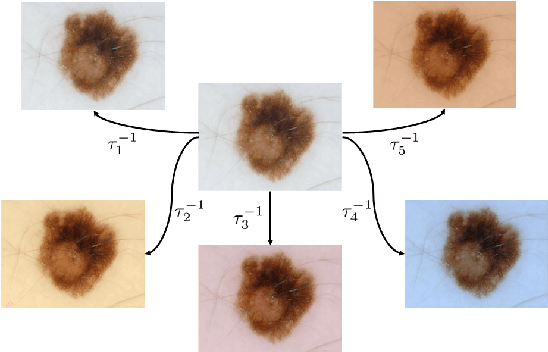
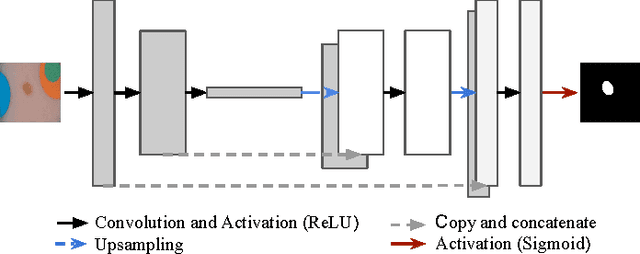
Abstract:Dermoscopic skin images are often obtained with different imaging devices, under varying acquisition conditions. In this work, instead of attempting to perform intensity and color normalization, we propose to leverage computational color constancy techniques to build an artificial data augmentation technique suitable for this kind of images. Specifically, we apply the \emph{shades of gray} color constancy technique to color-normalize the entire training set of images, while retaining the estimated illuminants. We then draw one sample from the distribution of training set illuminants and apply it on the normalized image. We employ this technique for training two deep convolutional neural networks for the tasks of skin lesion segmentation and skin lesion classification, in the context of the ISIC 2017 challenge and without using any external dermatologic image set. Our results on the validation set are promising, and will be supplemented with extended results on the hidden test set when available.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge