Lauren J O'Donnell
A Novel Deep Learning Tractography Fiber Clustering Framework for Functionally Consistent White Matter Parcellation Using Multimodal Diffusion MRI and Functional MRI
Nov 04, 2024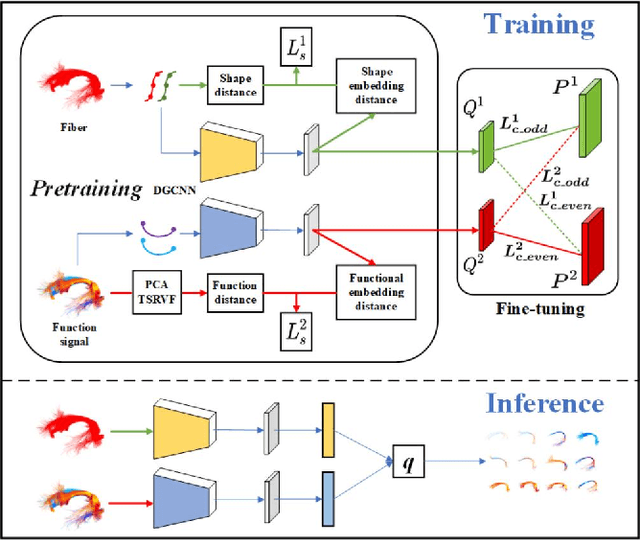
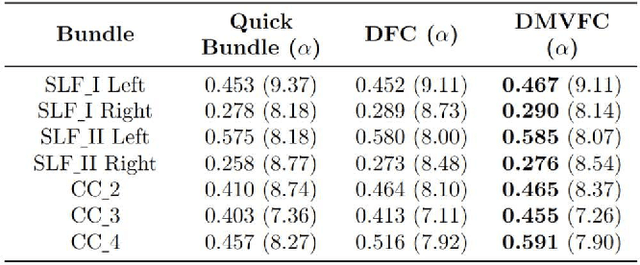
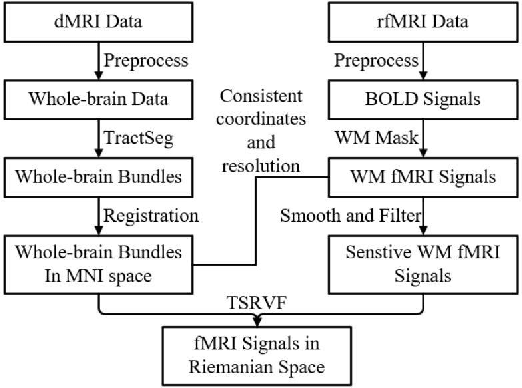
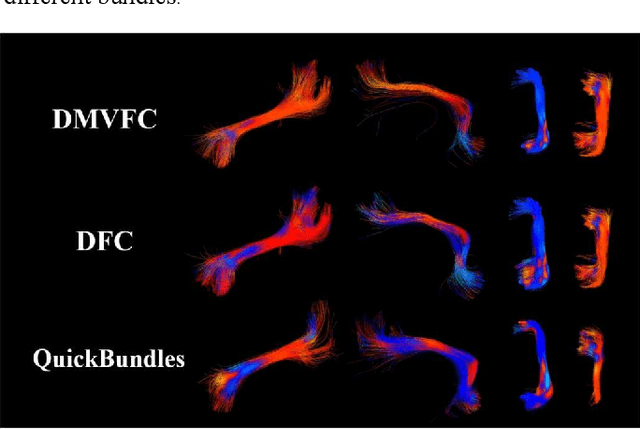
Abstract:Tractography fiber clustering using diffusion MRI (dMRI) is a crucial strategy for white matter (WM) parcellation. Current methods primarily use the geometric information of fibers (i.e., the spatial trajectories) to group similar fibers into clusters, overlooking the important functional signals present along the fiber tracts. There is increasing evidence that neural activity in the WM can be measured using functional MRI (fMRI), offering potentially valuable multimodal information for fiber clustering. In this paper, we develop a novel deep learning fiber clustering framework, namely Deep Multi-view Fiber Clustering (DMVFC), that uses joint dMRI and fMRI data to enable functionally consistent WM parcellation. DMVFC can effectively integrate the geometric characteristics of the WM fibers with the fMRI BOLD signals along the fiber tracts. It includes two major components: 1) a multi-view pretraining module to compute embedding features from fiber geometric information and functional signals separately, and 2) a collaborative fine-tuning module to simultaneously refine the two kinds of embeddings. In the experiments, we compare DMVFC with two state-of-the-art fiber clustering methods and demonstrate superior performance in achieving functionally meaningful and consistent WM parcellation results.
DeepRGVP: A Novel Microstructure-Informed Supervised Contrastive Learning Framework for Automated Identification Of The Retinogeniculate Pathway Using dMRI Tractography
Nov 15, 2022



Abstract:The retinogeniculate pathway (RGVP) is responsible for carrying visual information from the retina to the lateral geniculate nucleus. Identification and visualization of the RGVP are important in studying the anatomy of the visual system and can inform treatment of related brain diseases. Diffusion MRI (dMRI) tractography is an advanced imaging method that uniquely enables in vivo mapping of the 3D trajectory of the RGVP. Currently, identification of the RGVP from tractography data relies on expert (manual) selection of tractography streamlines, which is time-consuming, has high clinical and expert labor costs, and affected by inter-observer variability. In this paper, we present what we believe is the first deep learning framework, namely DeepRGVP, to enable fast and accurate identification of the RGVP from dMRI tractography data. We design a novel microstructure-informed supervised contrastive learning method that leverages both streamline label and tissue microstructure information to determine positive and negative pairs. We propose a simple and successful streamline-level data augmentation method to address highly imbalanced training data, where the number of RGVP streamlines is much lower than that of non-RGVP streamlines. We perform comparisons with several state-of-the-art deep learning methods that were designed for tractography parcellation, and we show superior RGVP identification results using DeepRGVP.
TractoFormer: A Novel Fiber-level Whole Brain Tractography Analysis Framework Using Spectral Embedding and Vision Transformers
Jul 11, 2022



Abstract:Diffusion MRI tractography is an advanced imaging technique for quantitative mapping of the brain's structural connectivity. Whole brain tractography (WBT) data contains over hundreds of thousands of individual fiber streamlines (estimated brain connections), and this data is usually parcellated to create compact representations for data analysis applications such as disease classification. In this paper, we propose a novel parcellation-free WBT analysis framework, TractoFormer, that leverages tractography information at the level of individual fiber streamlines and provides a natural mechanism for interpretation of results using the attention mechanism of transformers. TractoFormer includes two main contributions. First, we propose a novel and simple 2D image representation of WBT, TractoEmbedding, to encode 3D fiber spatial relationships and any feature of interest that can be computed from individual fibers (such as FA or MD). Second, we design a network based on vision transformers (ViTs) that includes: 1) data augmentation to overcome model overfitting on small datasets, 2) identification of discriminative fibers for interpretation of results, and 3) ensemble learning to leverage fiber information from different brain regions. In a synthetic data experiment, TractoFormer successfully identifies discriminative fibers with simulated group differences. In a disease classification experiment comparing several methods, TractoFormer achieves the highest accuracy in classifying schizophrenia vs control. Discriminative fibers are identified in left hemispheric frontal and parietal superficial white matter regions, which have previously been shown to be affected in schizophrenia patients.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge