Kunal Lillaney
PACSET (Packed Serialized Trees): Reducing Inference Latency for Tree Ensemble Deployment
Nov 10, 2020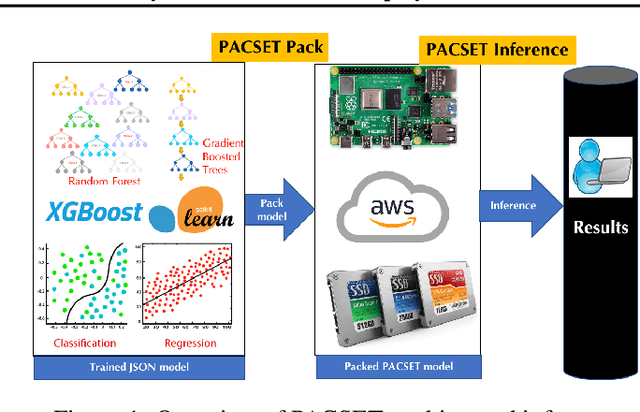

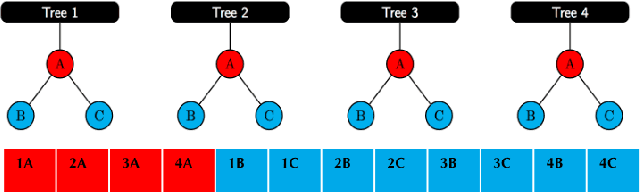

Abstract:We present methods to serialize and deserialize tree ensembles that optimize inference latency when models are not already loaded into memory. This arises whenever models are larger than memory, but also systematically when models are deployed on low-resource devices, such as in the Internet of Things, or run as Web micro-services where resources are allocated on demand. Our packed serialized trees (PACSET) encode reference locality in the layout of a tree ensemble using principles from external memory algorithms. The layout interleaves correlated nodes across multiple trees, uses leaf cardinality to collocate the nodes on the most popular paths and is optimized for the I/O blocksize. The result is that each I/O yields a higher fraction of useful data, leading to a 2-6 times reduction in classification latency for interactive workloads.
A Large Deformation Diffeomorphic Approach to Registration of CLARITY Images via Mutual Information
Aug 11, 2017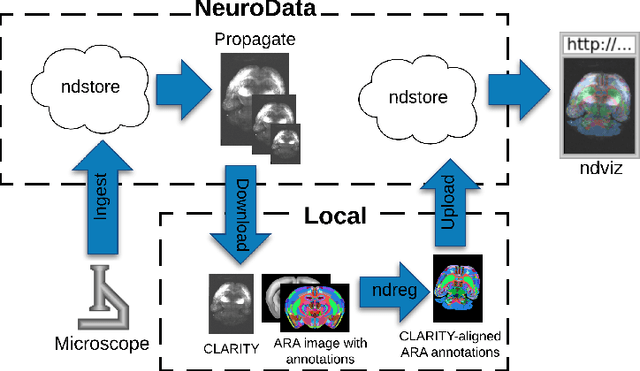
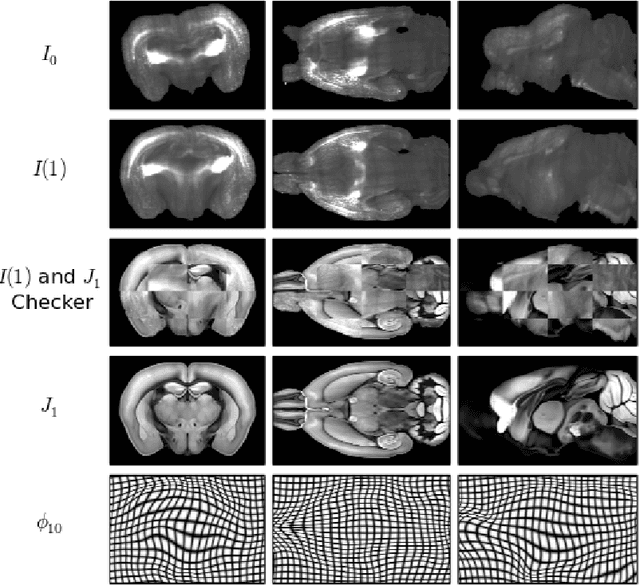
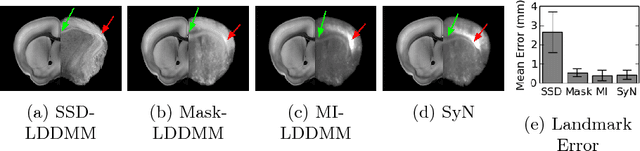
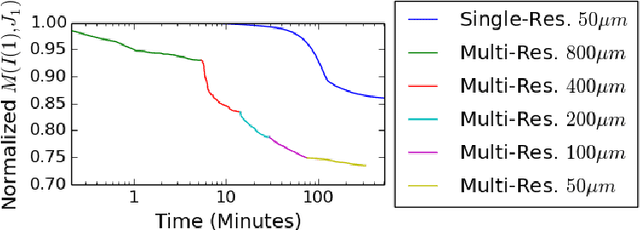
Abstract:CLARITY is a method for converting biological tissues into translucent and porous hydrogel-tissue hybrids. This facilitates interrogation with light sheet microscopy and penetration of molecular probes while avoiding physical slicing. In this work, we develop a pipeline for registering CLARIfied mouse brains to an annotated brain atlas. Due to the novelty of this microscopy technique it is impractical to use absolute intensity values to align these images to existing standard atlases. Thus we adopt a large deformation diffeomorphic approach for registering images via mutual information matching. Furthermore we show how a cascaded multi-resolution approach can improve registration quality while reducing algorithm run time. As acquired image volumes were over a terabyte in size, they were far too large for work on personal computers. Therefore the NeuroData computational infrastructure was deployed for multi-resolution storage and visualization of these images and aligned annotations on the web.
An Automated Images-to-Graphs Framework for High Resolution Connectomics
Apr 30, 2015


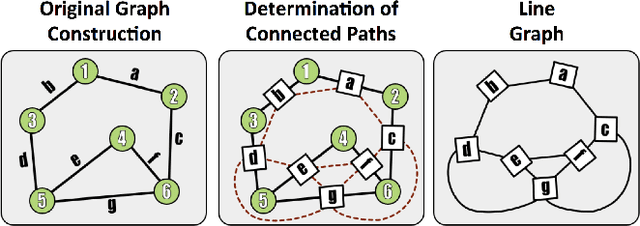
Abstract:Reconstructing a map of neuronal connectivity is a critical challenge in contemporary neuroscience. Recent advances in high-throughput serial section electron microscopy (EM) have produced massive 3D image volumes of nanoscale brain tissue for the first time. The resolution of EM allows for individual neurons and their synaptic connections to be directly observed. Recovering neuronal networks by manually tracing each neuronal process at this scale is unmanageable, and therefore researchers are developing automated image processing modules. Thus far, state-of-the-art algorithms focus only on the solution to a particular task (e.g., neuron segmentation or synapse identification). In this manuscript we present the first fully automated images-to-graphs pipeline (i.e., a pipeline that begins with an imaged volume of neural tissue and produces a brain graph without any human interaction). To evaluate overall performance and select the best parameters and methods, we also develop a metric to assess the quality of the output graphs. We evaluate a set of algorithms and parameters, searching possible operating points to identify the best available brain graph for our assessment metric. Finally, we deploy a reference end-to-end version of the pipeline on a large, publicly available data set. This provides a baseline result and framework for community analysis and future algorithm development and testing. All code and data derivatives have been made publicly available toward eventually unlocking new biofidelic computational primitives and understanding of neuropathologies.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge