Judy W Gichoya
The Limits of Fair Medical Imaging AI In The Wild
Dec 11, 2023Abstract:As artificial intelligence (AI) rapidly approaches human-level performance in medical imaging, it is crucial that it does not exacerbate or propagate healthcare disparities. Prior research has established AI's capacity to infer demographic data from chest X-rays, leading to a key concern: do models using demographic shortcuts have unfair predictions across subpopulations? In this study, we conduct a thorough investigation into the extent to which medical AI utilizes demographic encodings, focusing on potential fairness discrepancies within both in-distribution training sets and external test sets. Our analysis covers three key medical imaging disciplines: radiology, dermatology, and ophthalmology, and incorporates data from six global chest X-ray datasets. We confirm that medical imaging AI leverages demographic shortcuts in disease classification. While correcting shortcuts algorithmically effectively addresses fairness gaps to create "locally optimal" models within the original data distribution, this optimality is not true in new test settings. Surprisingly, we find that models with less encoding of demographic attributes are often most "globally optimal", exhibiting better fairness during model evaluation in new test environments. Our work establishes best practices for medical imaging models which maintain their performance and fairness in deployments beyond their initial training contexts, underscoring critical considerations for AI clinical deployments across populations and sites.
Reading Race: AI Recognises Patient's Racial Identity In Medical Images
Jul 21, 2021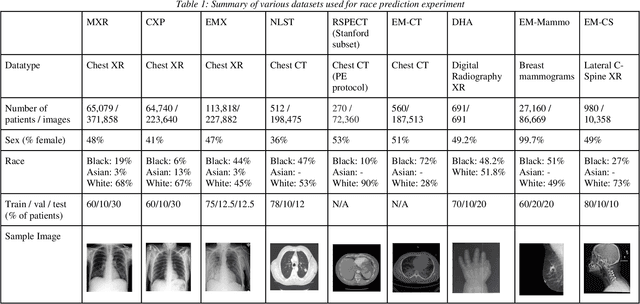
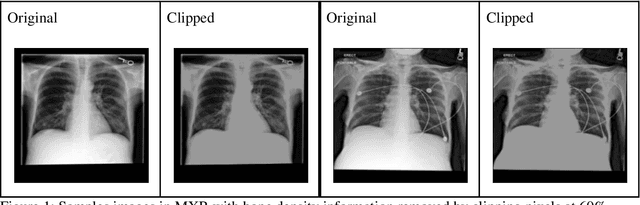
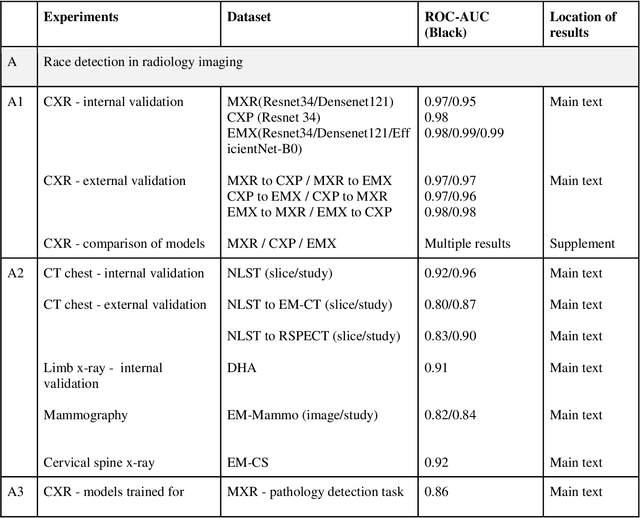
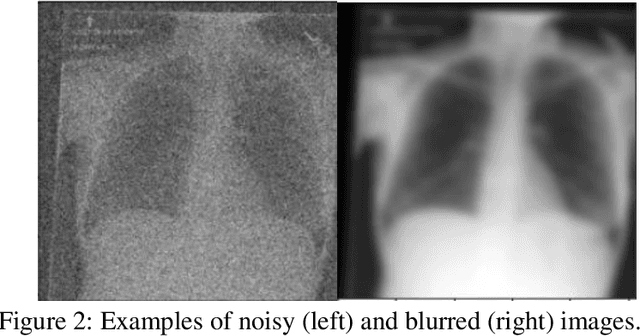
Abstract:Background: In medical imaging, prior studies have demonstrated disparate AI performance by race, yet there is no known correlation for race on medical imaging that would be obvious to the human expert interpreting the images. Methods: Using private and public datasets we evaluate: A) performance quantification of deep learning models to detect race from medical images, including the ability of these models to generalize to external environments and across multiple imaging modalities, B) assessment of possible confounding anatomic and phenotype population features, such as disease distribution and body habitus as predictors of race, and C) investigation into the underlying mechanism by which AI models can recognize race. Findings: Standard deep learning models can be trained to predict race from medical images with high performance across multiple imaging modalities. Our findings hold under external validation conditions, as well as when models are optimized to perform clinically motivated tasks. We demonstrate this detection is not due to trivial proxies or imaging-related surrogate covariates for race, such as underlying disease distribution. Finally, we show that performance persists over all anatomical regions and frequency spectrum of the images suggesting that mitigation efforts will be challenging and demand further study. Interpretation: We emphasize that model ability to predict self-reported race is itself not the issue of importance. However, our findings that AI can trivially predict self-reported race -- even from corrupted, cropped, and noised medical images -- in a setting where clinical experts cannot, creates an enormous risk for all model deployments in medical imaging: if an AI model secretly used its knowledge of self-reported race to misclassify all Black patients, radiologists would not be able to tell using the same data the model has access to.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge