John L. Burns
Reading Race: AI Recognises Patient's Racial Identity In Medical Images
Jul 21, 2021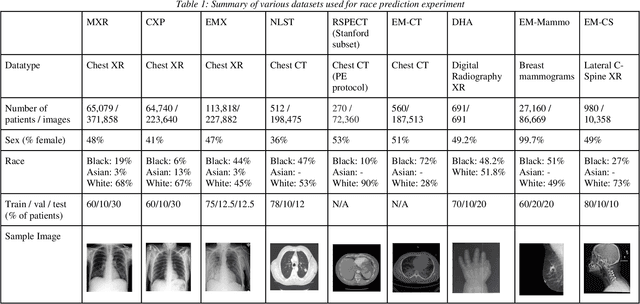
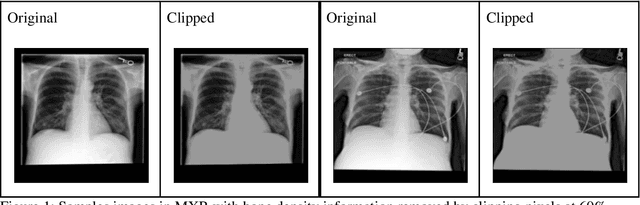
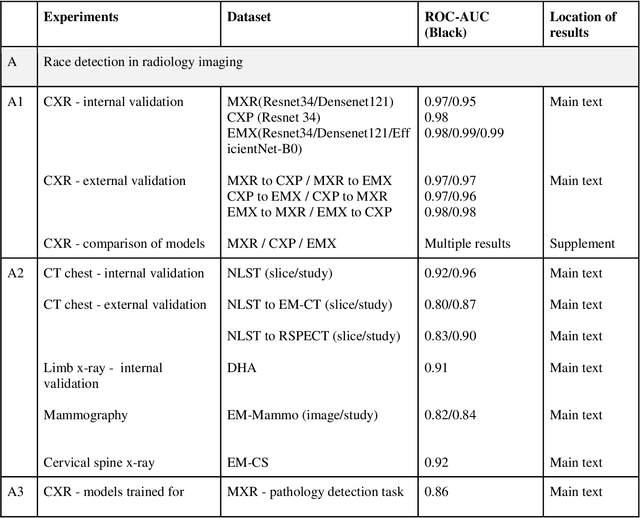
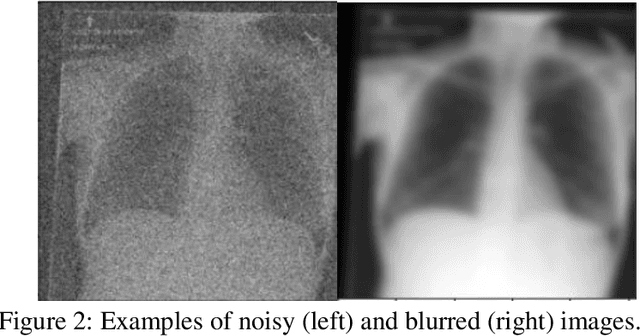
Abstract:Background: In medical imaging, prior studies have demonstrated disparate AI performance by race, yet there is no known correlation for race on medical imaging that would be obvious to the human expert interpreting the images. Methods: Using private and public datasets we evaluate: A) performance quantification of deep learning models to detect race from medical images, including the ability of these models to generalize to external environments and across multiple imaging modalities, B) assessment of possible confounding anatomic and phenotype population features, such as disease distribution and body habitus as predictors of race, and C) investigation into the underlying mechanism by which AI models can recognize race. Findings: Standard deep learning models can be trained to predict race from medical images with high performance across multiple imaging modalities. Our findings hold under external validation conditions, as well as when models are optimized to perform clinically motivated tasks. We demonstrate this detection is not due to trivial proxies or imaging-related surrogate covariates for race, such as underlying disease distribution. Finally, we show that performance persists over all anatomical regions and frequency spectrum of the images suggesting that mitigation efforts will be challenging and demand further study. Interpretation: We emphasize that model ability to predict self-reported race is itself not the issue of importance. However, our findings that AI can trivially predict self-reported race -- even from corrupted, cropped, and noised medical images -- in a setting where clinical experts cannot, creates an enormous risk for all model deployments in medical imaging: if an AI model secretly used its knowledge of self-reported race to misclassify all Black patients, radiologists would not be able to tell using the same data the model has access to.
A Prospective Observational Study to Investigate Performance of a Chest X-ray Artificial Intelligence Diagnostic Support Tool Across 12 U.S. Hospitals
Jun 07, 2021Abstract:Importance: An artificial intelligence (AI)-based model to predict COVID-19 likelihood from chest x-ray (CXR) findings can serve as an important adjunct to accelerate immediate clinical decision making and improve clinical decision making. Despite significant efforts, many limitations and biases exist in previously developed AI diagnostic models for COVID-19. Utilizing a large set of local and international CXR images, we developed an AI model with high performance on temporal and external validation. Conclusions and Relevance: AI-based diagnostic tools may serve as an adjunct, but not replacement, for clinical decision support of COVID-19 diagnosis, which largely hinges on exposure history, signs, and symptoms. While AI-based tools have not yet reached full diagnostic potential in COVID-19, they may still offer valuable information to clinicians taken into consideration along with clinical signs and symptoms.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge