Jens Kuhle
GAMER-MRIL identifies Disability-Related Brain Changes in Multiple Sclerosis
Aug 15, 2023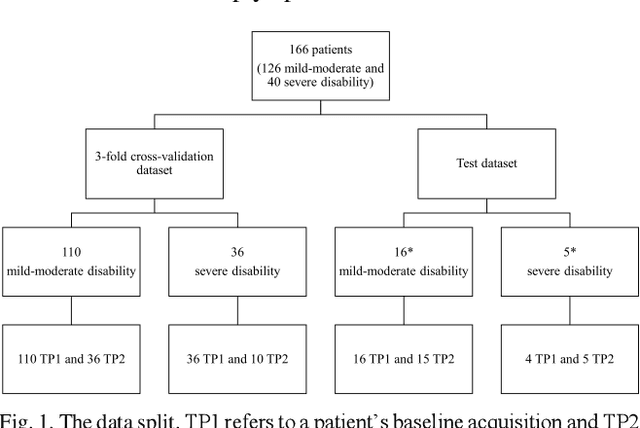
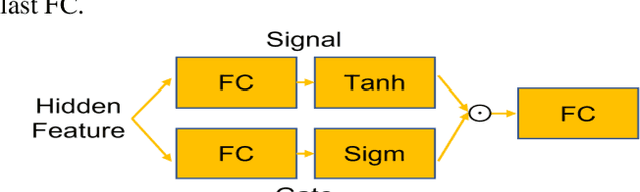
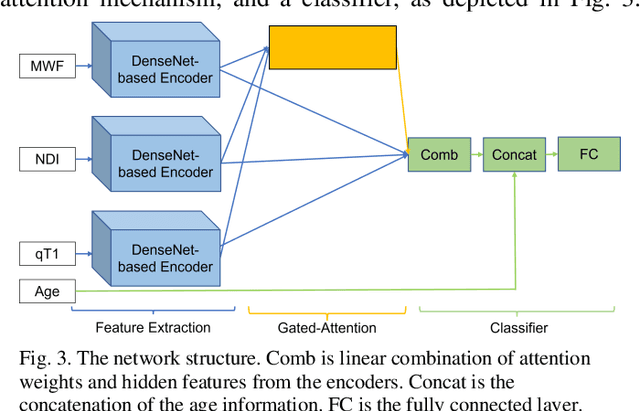
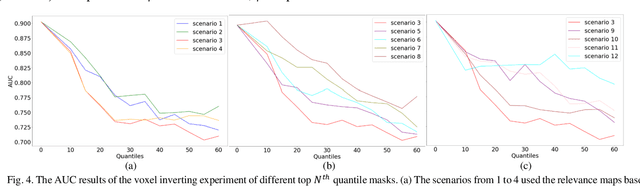
Abstract:Objective: Identifying disability-related brain changes is important for multiple sclerosis (MS) patients. Currently, there is no clear understanding about which pathological features drive disability in single MS patients. In this work, we propose a novel comprehensive approach, GAMER-MRIL, leveraging whole-brain quantitative MRI (qMRI), convolutional neural network (CNN), and an interpretability method from classifying MS patients with severe disability to investigating relevant pathological brain changes. Methods: One-hundred-sixty-six MS patients underwent 3T MRI acquisitions. qMRI informative of microstructural brain properties was reconstructed, including quantitative T1 (qT1), myelin water fraction (MWF), and neurite density index (NDI). To fully utilize the qMRI, GAMER-MRIL extended a gated-attention-based CNN (GAMER-MRI), which was developed to select patch-based qMRI important for a given task/question, to the whole-brain image. To find out disability-related brain regions, GAMER-MRIL modified a structure-aware interpretability method, Layer-wise Relevance Propagation (LRP), to incorporate qMRI. Results: The test performance was AUC=0.885. qT1 was the most sensitive measure related to disability, followed by NDI. The proposed LRP approach obtained more specifically relevant regions than other interpretability methods, including the saliency map, the integrated gradients, and the original LRP. The relevant regions included the corticospinal tract, where average qT1 and NDI significantly correlated with patients' disability scores ($\rho$=-0.37 and 0.44). Conclusion: These results demonstrated that GAMER-MRIL can classify patients with severe disability using qMRI and subsequently identify brain regions potentially important to the integrity of the mobile function. Significance: GAMER-MRIL holds promise for developing biomarkers and increasing clinicians' trust in NN.
Learn to Ignore: Domain Adaptation for Multi-Site MRI Analysis
Oct 13, 2021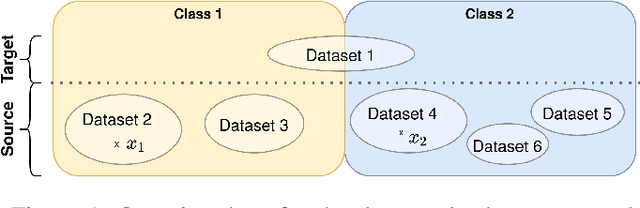
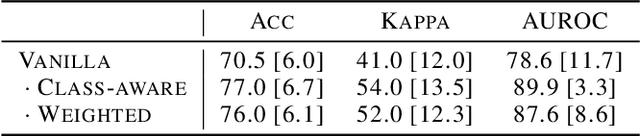
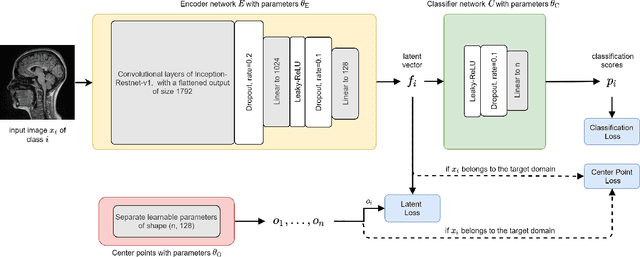
Abstract:Limited availability of large image datasets is a major issue in the development of accurate and generalizable machine learning methods in medicine. The limitations in the amount of data are mainly due to the use of different acquisition protocols, different hardware, and data privacy. At the same time, training a classification model on a small dataset leads to a poor generalization quality of the model. To overcome this issue, a combination of various image datasets of different provenance is often used, e.g., multi-site studies. However, if an additional dataset does not include all classes of the task, the learning of the classification model can be biased to the device or place of acquisition. This is especially the case for Magnetic Resonance (MR) images, where different MR scanners introduce a bias that limits the performance of the model. In this paper, we present a novel method that learns to ignore the scanner-related features present in the images, while learning features relevant for the classification task. We focus on a real-world scenario, where only a small dataset provides images of all classes. We exploit this circumstance by introducing specific additional constraints on the latent space, which lead the focus on disease-related rather than scanner-specific features. Our method Learn to Ignore outperforms state-of-the-art domain adaptation methods on a multi-site MRI dataset on a classification task between Multiple Sclerosis patients and healthy subjects.
Longitudinal modeling of MS patient trajectories improves predictions of disability progression
Nov 09, 2020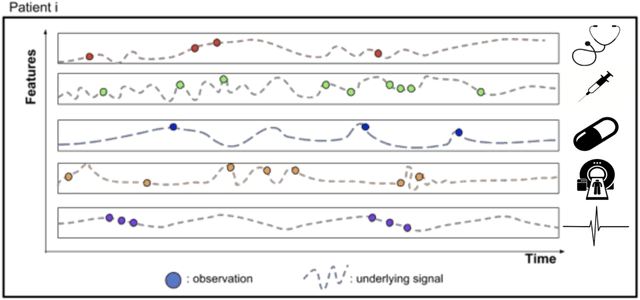
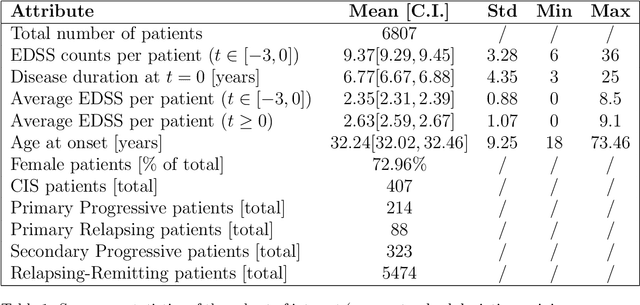
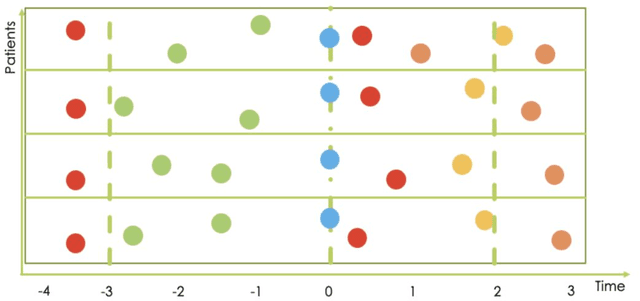
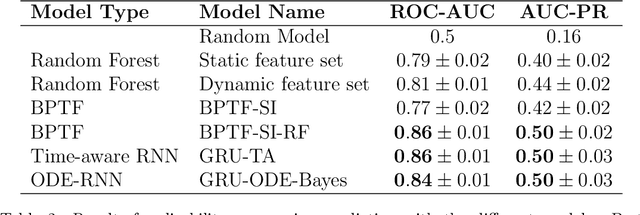
Abstract:Research in Multiple Sclerosis (MS) has recently focused on extracting knowledge from real-world clinical data sources. This type of data is more abundant than data produced during clinical trials and potentially more informative about real-world clinical practice. However, this comes at the cost of less curated and controlled data sets. In this work, we address the task of optimally extracting information from longitudinal patient data in the real-world setting with a special focus on the sporadic sampling problem. Using the MSBase registry, we show that with machine learning methods suited for patient trajectories modeling, such as recurrent neural networks and tensor factorization, we can predict disability progression of patients in a two-year horizon with an ROC-AUC of 0.86, which represents a 33% decrease in the ranking pair error (1-AUC) compared to reference methods using static clinical features. Compared to the models available in the literature, this work uses the most complete patient history for MS disease progression prediction.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge