Jeffrey Caterino
SepsisCalc: Integrating Clinical Calculators into Early Sepsis Prediction via Dynamic Temporal Graph Construction
Dec 31, 2024



Abstract:Sepsis is an organ dysfunction caused by a deregulated immune response to an infection. Early sepsis prediction and identification allow for timely intervention, leading to improved clinical outcomes. Clinical calculators (e.g., the six-organ dysfunction assessment of SOFA) play a vital role in sepsis identification within clinicians' workflow, providing evidence-based risk assessments essential for sepsis diagnosis. However, artificial intelligence (AI) sepsis prediction models typically generate a single sepsis risk score without incorporating clinical calculators for assessing organ dysfunctions, making the models less convincing and transparent to clinicians. To bridge the gap, we propose to mimic clinicians' workflow with a novel framework SepsisCalc to integrate clinical calculators into the predictive model, yielding a clinically transparent and precise model for utilization in clinical settings. Practically, clinical calculators usually combine information from multiple component variables in Electronic Health Records (EHR), and might not be applicable when the variables are (partially) missing. We mitigate this issue by representing EHRs as temporal graphs and integrating a learning module to dynamically add the accurately estimated calculator to the graphs. Experimental results on real-world datasets show that the proposed model outperforms state-of-the-art methods on sepsis prediction tasks. Moreover, we developed a system to identify organ dysfunctions and potential sepsis risks, providing a human-AI interaction tool for deployment, which can help clinicians understand the prediction outputs and prepare timely interventions for the corresponding dysfunctions, paving the way for actionable clinical decision-making support for early intervention.
SepsisLab: Early Sepsis Prediction with Uncertainty Quantification and Active Sensing
Jul 24, 2024Abstract:Sepsis is the leading cause of in-hospital mortality in the USA. Early sepsis onset prediction and diagnosis could significantly improve the survival of sepsis patients. Existing predictive models are usually trained on high-quality data with few missing information, while missing values widely exist in real-world clinical scenarios (especially in the first hours of admissions to the hospital), which causes a significant decrease in accuracy and an increase in uncertainty for the predictive models. The common method to handle missing values is imputation, which replaces the unavailable variables with estimates from the observed data. The uncertainty of imputation results can be propagated to the sepsis prediction outputs, which have not been studied in existing works on either sepsis prediction or uncertainty quantification. In this study, we first define such propagated uncertainty as the variance of prediction output and then introduce uncertainty propagation methods to quantify the propagated uncertainty. Moreover, for the potential high-risk patients with low confidence due to limited observations, we propose a robust active sensing algorithm to increase confidence by actively recommending clinicians to observe the most informative variables. We validate the proposed models in both publicly available data (i.e., MIMIC-III and AmsterdamUMCdb) and proprietary data in The Ohio State University Wexner Medical Center (OSUWMC). The experimental results show that the propagated uncertainty is dominant at the beginning of admissions to hospitals and the proposed algorithm outperforms state-of-the-art active sensing methods. Finally, we implement a SepsisLab system for early sepsis prediction and active sensing based on our pre-trained models. Clinicians and potential sepsis patients can benefit from the system in early prediction and diagnosis of sepsis.
Rethinking Human-AI Collaboration in Complex Medical Decision Making: A Case Study in Sepsis Diagnosis
Sep 17, 2023



Abstract:Today's AI systems for medical decision support often succeed on benchmark datasets in research papers but fail in real-world deployment. This work focuses on the decision making of sepsis, an acute life-threatening systematic infection that requires an early diagnosis with high uncertainty from the clinician. Our aim is to explore the design requirements for AI systems that can support clinical experts in making better decisions for the early diagnosis of sepsis. The study begins with a formative study investigating why clinical experts abandon an existing AI-powered Sepsis predictive module in their electrical health record (EHR) system. We argue that a human-centered AI system needs to support human experts in the intermediate stages of a medical decision-making process (e.g., generating hypotheses or gathering data), instead of focusing only on the final decision. Therefore, we build SepsisLab based on a state-of-the-art AI algorithm and extend it to predict the future projection of sepsis development, visualize the prediction uncertainty, and propose actionable suggestions (i.e., which additional laboratory tests can be collected) to reduce such uncertainty. Through heuristic evaluation with six clinicians using our prototype system, we demonstrate that SepsisLab enables a promising human-AI collaboration paradigm for the future of AI-assisted sepsis diagnosis and other high-stakes medical decision making.
Deconfounding Actor-Critic Network with Policy Adaptation for Dynamic Treatment Regimes
May 31, 2022

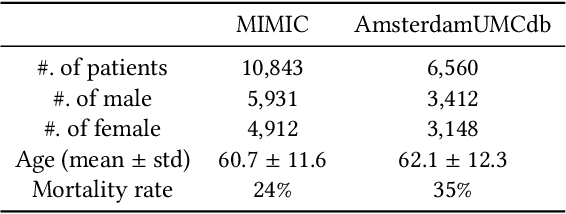

Abstract:Despite intense efforts in basic and clinical research, an individualized ventilation strategy for critically ill patients remains a major challenge. Recently, dynamic treatment regime (DTR) with reinforcement learning (RL) on electronic health records (EHR) has attracted interest from both the healthcare industry and machine learning research community. However, most learned DTR policies might be biased due to the existence of confounders. Although some treatment actions non-survivors received may be helpful, if confounders cause the mortality, the training of RL models guided by long-term outcomes (e.g., 90-day mortality) would punish those treatment actions causing the learned DTR policies to be suboptimal. In this study, we develop a new deconfounding actor-critic network (DAC) to learn optimal DTR policies for patients. To alleviate confounding issues, we incorporate a patient resampling module and a confounding balance module into our actor-critic framework. To avoid punishing the effective treatment actions non-survivors received, we design a short-term reward to capture patients' immediate health state changes. Combining short-term with long-term rewards could further improve the model performance. Moreover, we introduce a policy adaptation method to successfully transfer the learned model to new-source small-scale datasets. The experimental results on one semi-synthetic and two different real-world datasets show the proposed model outperforms the state-of-the-art models. The proposed model provides individualized treatment decisions for mechanical ventilation that could improve patient outcomes.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge