Heping Hu
A deep learning pipeline for localization, differentiation, and uncertainty estimation of liver lesions using multi-phasic and multi-sequence MRI
Oct 17, 2021



Abstract:Objectives: to propose a fully-automatic computer-aided diagnosis (CAD) solution for liver lesion characterization, with uncertainty estimation. Methods: we enrolled 400 patients who had either liver resection or a biopsy and was diagnosed with either hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma, or secondary metastasis, from 2006 to 2019. Each patient was scanned with T1WI, T2WI, T1WI venous phase (T2WI-V), T1WI arterial phase (T1WI-A), and DWI MRI sequences. We propose a fully-automatic deep CAD pipeline that localizes lesions from 3D MRI studies using key-slice parsing and provides a confidence measure for its diagnoses. We evaluate using five-fold cross validation and compare performance against three radiologists, including a senior hepatology radiologist, a junior hepatology radiologist and an abdominal radiologist. Results: the proposed CAD solution achieves a mean F1 score of 0.62, outperforming the abdominal radiologist (0.47), matching the junior hepatology radiologist (0.61), and underperforming the senior hepatology radiologist (0.68). The CAD system can informatively assess its diagnostic confidence, i.e., when only evaluating on the 70% most confident cases the mean f1 score and sensitivity at 80% specificity for HCC vs. others are boosted from 0.62 to 0.71 and 0.84 to 0.92, respectively. Conclusion: the proposed fully-automatic CAD solution can provide good diagnostic performance with informative confidence assessments in finding and discriminating liver lesions from MRI studies.
Hetero-Modal Learning and Expansive Consistency Constraints for Semi-Supervised Detection from Multi-Sequence Data
Mar 24, 2021



Abstract:Lesion detection serves a critical role in early diagnosis and has been well explored in recent years due to methodological advancesand increased data availability. However, the high costs of annotations hinder the collection of large and completely labeled datasets, motivating semi-supervised detection approaches. In this paper, we introduce mean teacher hetero-modal detection (MTHD), which addresses two important gaps in current semi-supervised detection. First, it is not obvious how to enforce unlabeled consistency constraints across the very different outputs of various detectors, which has resulted in various compromises being used in the state of the art. Using an anchor-free framework, MTHD formulates a mean teacher approach without such compromises, enforcing consistency on the soft-output of object centers and size. Second, multi-sequence data is often critical, e.g., for abdominal lesion detection, but unlabeled data is often missing sequences. To deal with this, MTHD incorporates hetero-modal learning in its framework. Unlike prior art, MTHD is able to incorporate an expansive set of consistency constraints that include geometric transforms and random sequence combinations. We train and evaluate MTHD on liver lesion detection using the largest MR lesion dataset to date (1099 patients with >5000 volumes). MTHD surpasses the best fully-supervised and semi-supervised competitors by 10.1% and 3.5%, respectively, in average sensitivity.
Fully-Automated Liver Tumor Localization and Characterization from Multi-Phase MR Volumes Using Key-Slice ROI Parsing: A Physician-Inspired Approach
Dec 15, 2020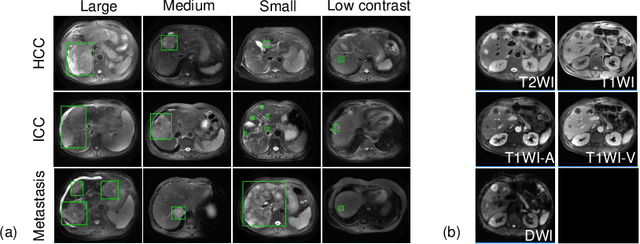
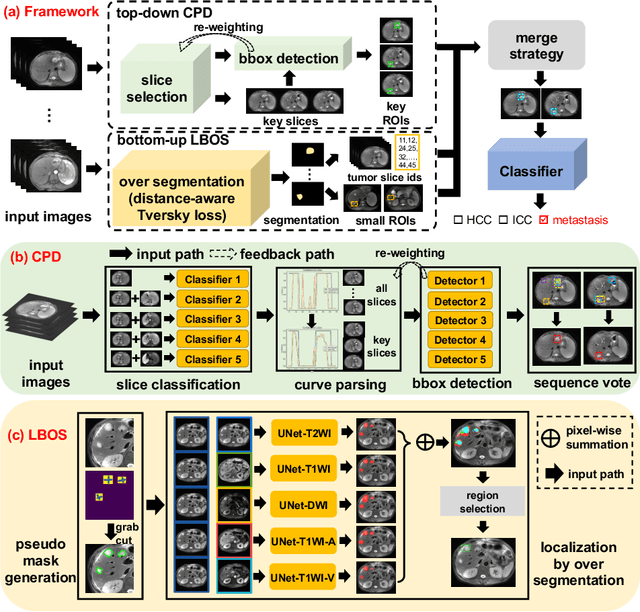
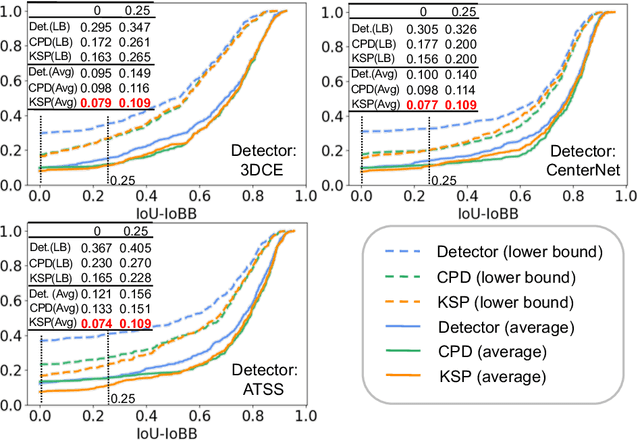
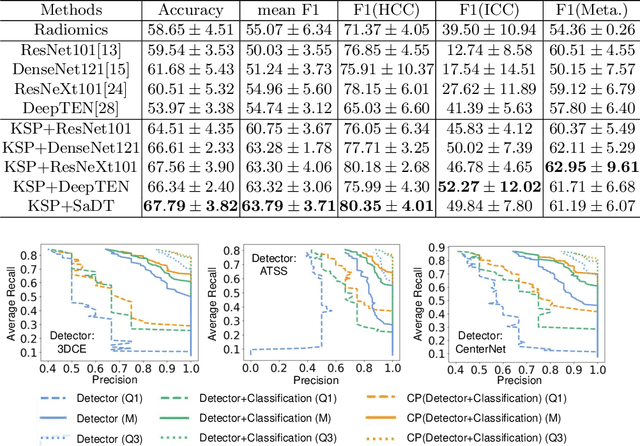
Abstract:Using radiological scans to identify liver tumors is crucial for proper patient treatment. This is highly challenging, as top radiologists only achieve F1 scores of roughly 80% (hepatocellular carcinoma (HCC) vs. others) with only moderate inter-rater agreement, even when using multi-phase magnetic resonance (MR) imagery. Thus, there is great impetus for computer-aided diagnosis (CAD) solutions. A critical challengeis to reliably parse a 3D MR volume to localize diagnosable regions of interest (ROI). In this paper, we break down this problem using a key-slice parser (KSP), which emulates physician workflows by first identifying key slices and then localize their corresponding key ROIs. Because performance demands are so extreme, (not to miss any key ROI),our KSP integrates complementary modules--top-down classification-plus-detection (CPD) and bottom-up localization-by-over-segmentation(LBOS). The CPD uses a curve-parsing and detection confidence to re-weight classifier confidences. The LBOS uses over-segmentation to flag CPD failure cases and provides its own ROIs. For scalability, LBOS is only weakly trained on pseudo-masks using a new distance-aware Tversky loss. We evaluate our approach on the largest multi-phase MR liver lesion test dataset to date (430 biopsy-confirmed patients). Experiments demonstrate that our KSP can localize diagnosable ROIs with high reliability (85% patients have an average overlap of >= 40% with the ground truth). Moreover, we achieve an HCC vs. others F1 score of 0.804, providing a fully-automated CAD solution comparable with top human physicians.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge