Harsh Bhatia
Center for Applied Scientific Computing, Lawrence Livermore National Laboratory
Identifying Orientation-specific Lipid-protein Fingerprints using Deep Learning
Jul 14, 2022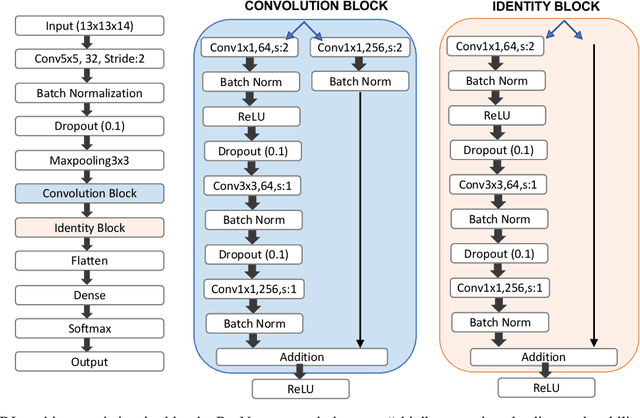
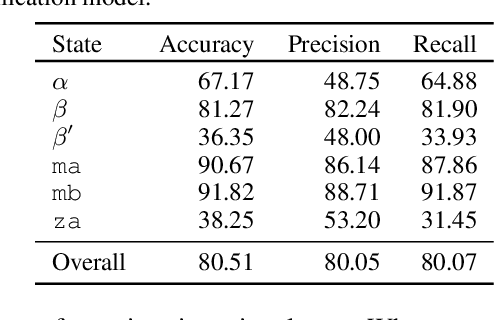
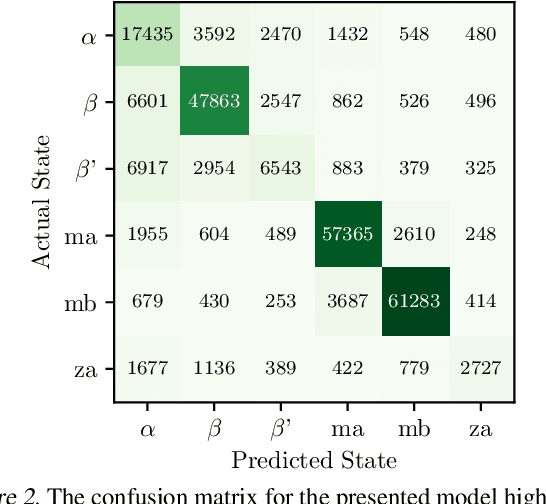
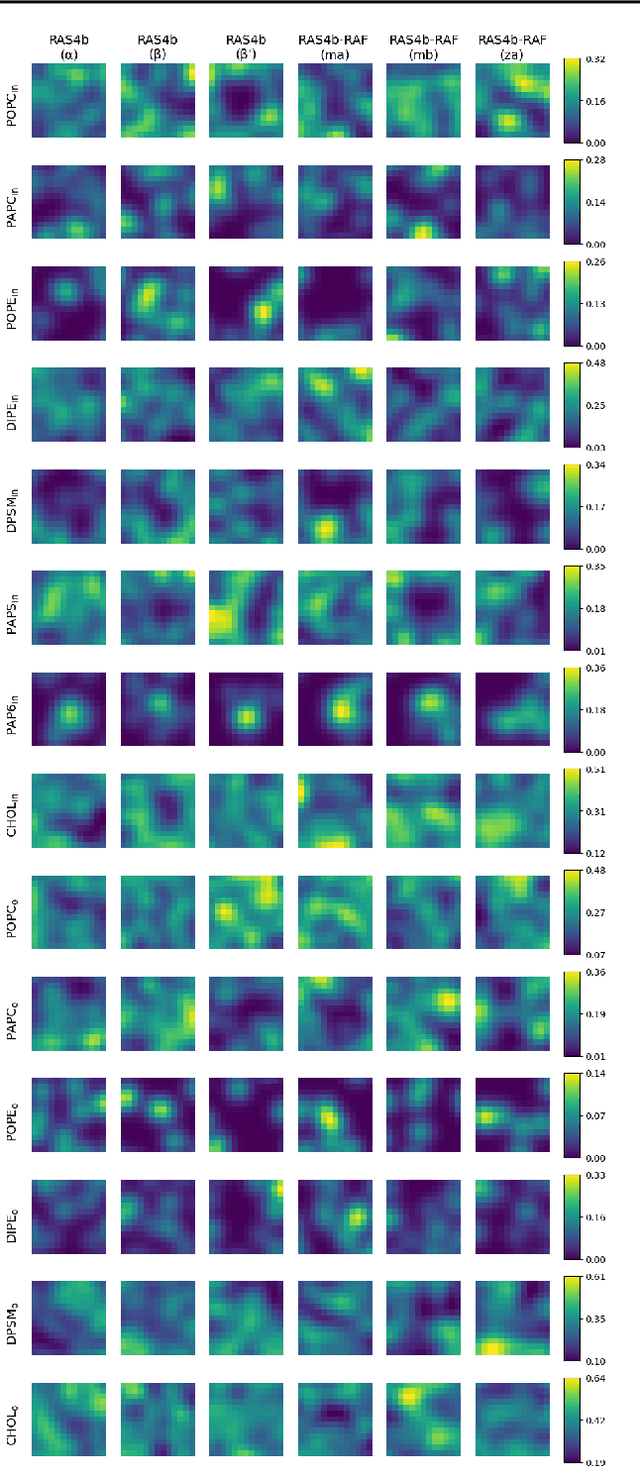
Abstract:Improved understanding of the relation between the behavior of RAS and RAF proteins and the local lipid environment in the cell membrane is critical for getting insights into the mechanisms underlying cancer formation. In this work, we employ deep learning (DL) to learn this relationship by predicting protein orientational states of RAS and RAS-RAF protein complexes with respect to the lipid membrane based on the lipid densities around the protein domains from coarse-grained (CG) molecular dynamics (MD) simulations. Our DL model can predict six protein states with an overall accuracy of over 80%. The findings of this work offer new insights into how the proteins modulate the lipid environment, which in turn may assist designing novel therapies to regulate such interactions in the mechanisms associated with cancer development.
Emerging Patterns in the Continuum Representation of Protein-Lipid Fingerprints
Jul 09, 2022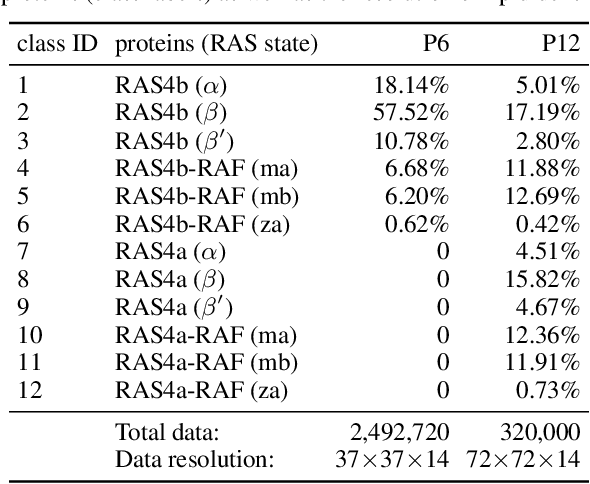
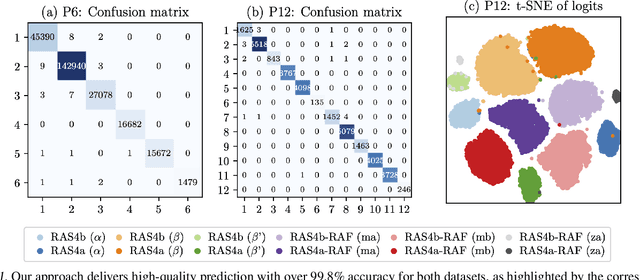
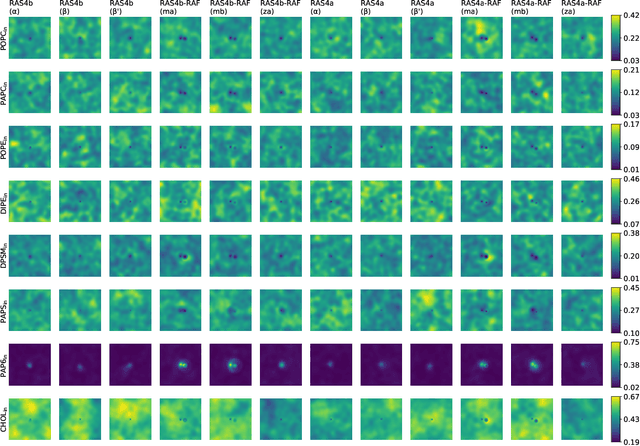
Abstract:Capturing intricate biological phenomena often requires multiscale modeling where coarse and inexpensive models are developed using limited components of expensive and high-fidelity models. Here, we consider such a multiscale framework in the context of cancer biology and address the challenge of evaluating the descriptive capabilities of a continuum model developed using 1-dimensional statistics from a molecular dynamics model. Using deep learning, we develop a highly predictive classification model that identifies complex and emergent behavior from the continuum model. With over 99.9% accuracy demonstrated for two simulations, our approach confirms the existence of protein-specific "lipid fingerprints", i.e. spatial rearrangements of lipids in response to proteins of interest. Through this demonstration, our model also provides external validation of the continuum model, affirms the value of such multiscale modeling, and can foster new insights through further analysis of these fingerprints.
Scalable Topological Data Analysis and Visualization for Evaluating Data-Driven Models in Scientific Applications
Jul 19, 2019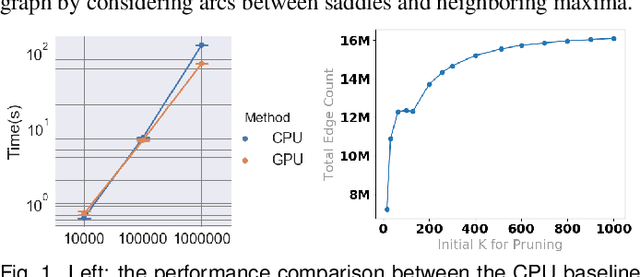
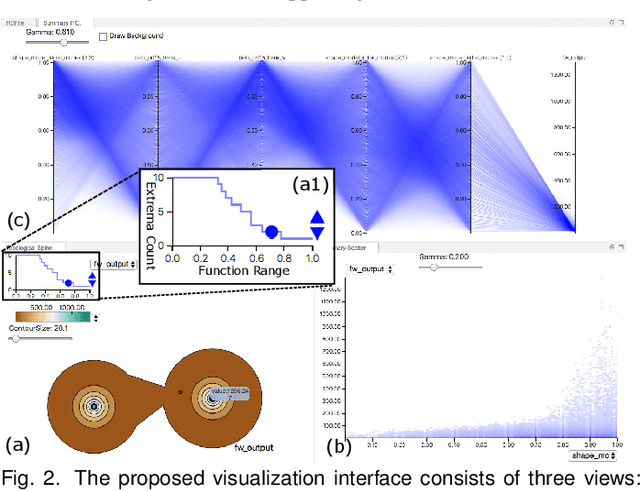
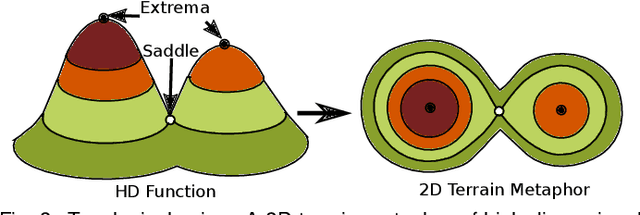
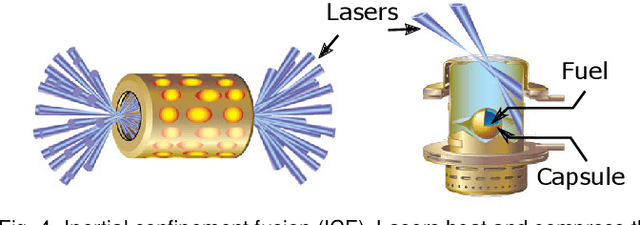
Abstract:With the rapid adoption of machine learning techniques for large-scale applications in science and engineering comes the convergence of two grand challenges in visualization. First, the utilization of black box models (e.g., deep neural networks) calls for advanced techniques in exploring and interpreting model behaviors. Second, the rapid growth in computing has produced enormous datasets that require techniques that can handle millions or more samples. Although some solutions to these interpretability challenges have been proposed, they typically do not scale beyond thousands of samples, nor do they provide the high-level intuition scientists are looking for. Here, we present the first scalable solution to explore and analyze high-dimensional functions often encountered in the scientific data analysis pipeline. By combining a new streaming neighborhood graph construction, the corresponding topology computation, and a novel data aggregation scheme, namely topology aware datacubes, we enable interactive exploration of both the topological and the geometric aspect of high-dimensional data. Following two use cases from high-energy-density (HED) physics and computational biology, we demonstrate how these capabilities have led to crucial new insights in both applications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge