Felice C. Lightstone
Physical & Life Sciences, Lawrence Livermore National Laboratory
Machine Learning-driven Multiscale MD Workflows: The Mini-MuMMI Experience
Jul 10, 2025Abstract:Computational models have become one of the prevalent methods to model complex phenomena. To accurately model complex interactions, such as detailed biomolecular interactions, scientists often rely on multiscale models comprised of several internal models operating at difference scales, ranging from microscopic to macroscopic length and time scales. Bridging the gap between different time and length scales has historically been challenging but the advent of newer machine learning (ML) approaches has shown promise for tackling that task. Multiscale models require massive amounts of computational power and a powerful workflow management system. Orchestrating ML-driven multiscale studies on parallel systems with thousands of nodes is challenging, the workflow must schedule, allocate and control thousands of simulations operating at different scales. Here, we discuss the massively parallel Multiscale Machine-Learned Modeling Infrastructure (MuMMI), a multiscale workflow management infrastructure, that can orchestrate thousands of molecular dynamics (MD) simulations operating at different timescales, spanning from millisecond to nanosecond. More specifically, we introduce a novel version of MuMMI called "mini-MuMMI". Mini-MuMMI is a curated version of MuMMI designed to run on modest HPC systems or even laptops whereas MuMMI requires larger HPC systems. We demonstrate mini-MuMMI utility by exploring RAS-RAF membrane interactions and discuss the different challenges behind the generalization of multiscale workflows and how mini-MuMMI can be leveraged to target a broader range of applications outside of MD and RAS-RAF interactions.
Evaluating Point-Prediction Uncertainties in Neural Networks for Drug Discovery
Oct 31, 2022



Abstract:Neural Network (NN) models provide potential to speed up the drug discovery process and reduce its failure rates. The success of NN models require uncertainty quantification (UQ) as drug discovery explores chemical space beyond the training data distribution. Standard NN models do not provide uncertainty information. Methods that combine Bayesian models with NN models address this issue, but are difficult to implement and more expensive to train. Some methods require changing the NN architecture or training procedure, limiting the selection of NN models. Moreover, predictive uncertainty can come from different sources. It is important to have the ability to separately model different types of predictive uncertainty, as the model can take assorted actions depending on the source of uncertainty. In this paper, we examine UQ methods that estimate different sources of predictive uncertainty for NN models aiming at drug discovery. We use our prior knowledge on chemical compounds to design the experiments. By utilizing a visualization method we create non-overlapping and chemically diverse partitions from a collection of chemical compounds. These partitions are used as training and test set splits to explore NN model uncertainty. We demonstrate how the uncertainties estimated by the selected methods describe different sources of uncertainty under different partitions and featurization schemes and the relationship to prediction error.
Identifying Orientation-specific Lipid-protein Fingerprints using Deep Learning
Jul 14, 2022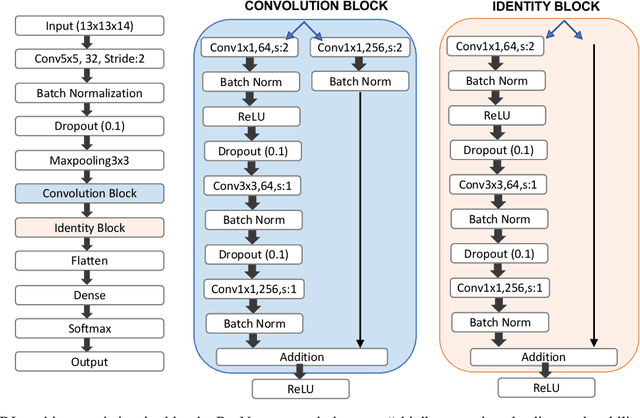
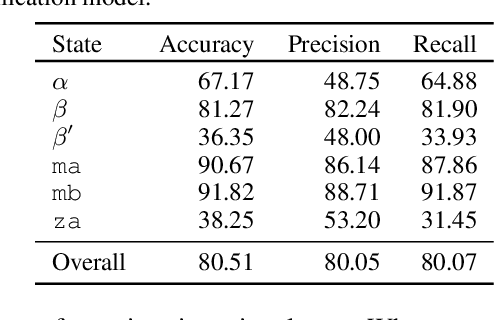
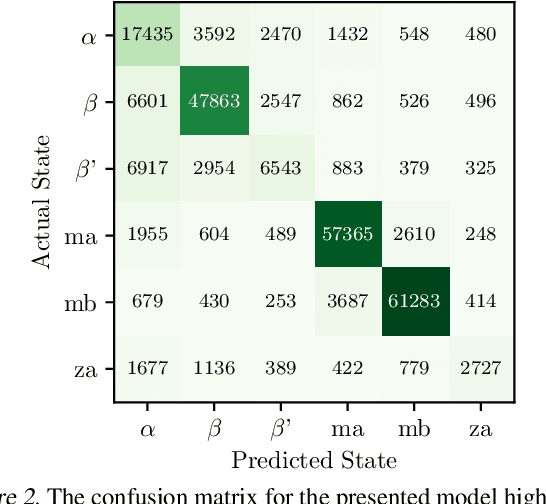
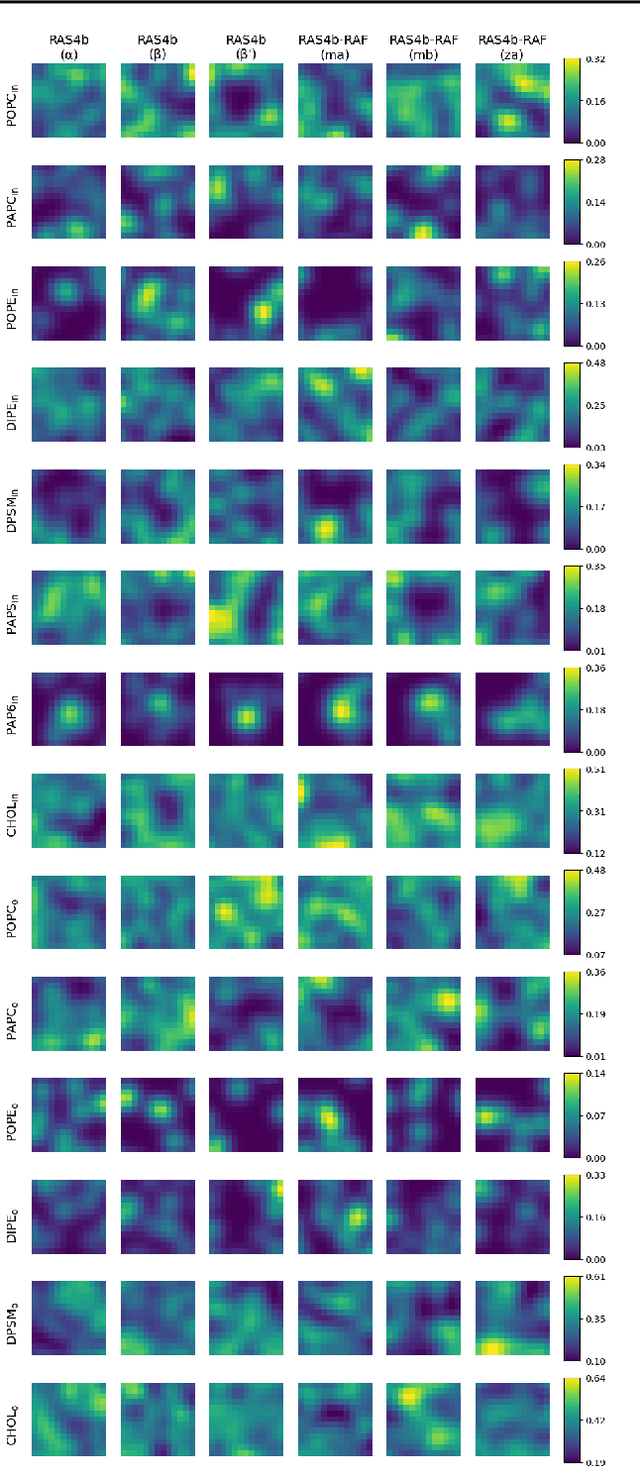
Abstract:Improved understanding of the relation between the behavior of RAS and RAF proteins and the local lipid environment in the cell membrane is critical for getting insights into the mechanisms underlying cancer formation. In this work, we employ deep learning (DL) to learn this relationship by predicting protein orientational states of RAS and RAS-RAF protein complexes with respect to the lipid membrane based on the lipid densities around the protein domains from coarse-grained (CG) molecular dynamics (MD) simulations. Our DL model can predict six protein states with an overall accuracy of over 80%. The findings of this work offer new insights into how the proteins modulate the lipid environment, which in turn may assist designing novel therapies to regulate such interactions in the mechanisms associated with cancer development.
High-Throughput Virtual Screening of Small Molecule Inhibitors for SARS-CoV-2 Protein Targets with Deep Fusion Models
Apr 09, 2021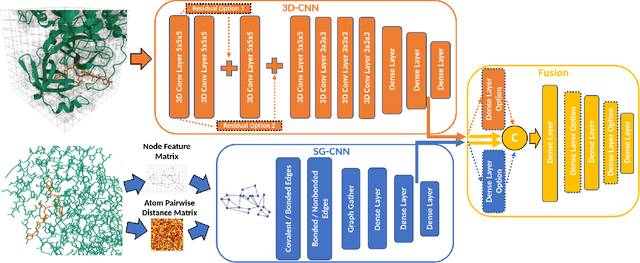
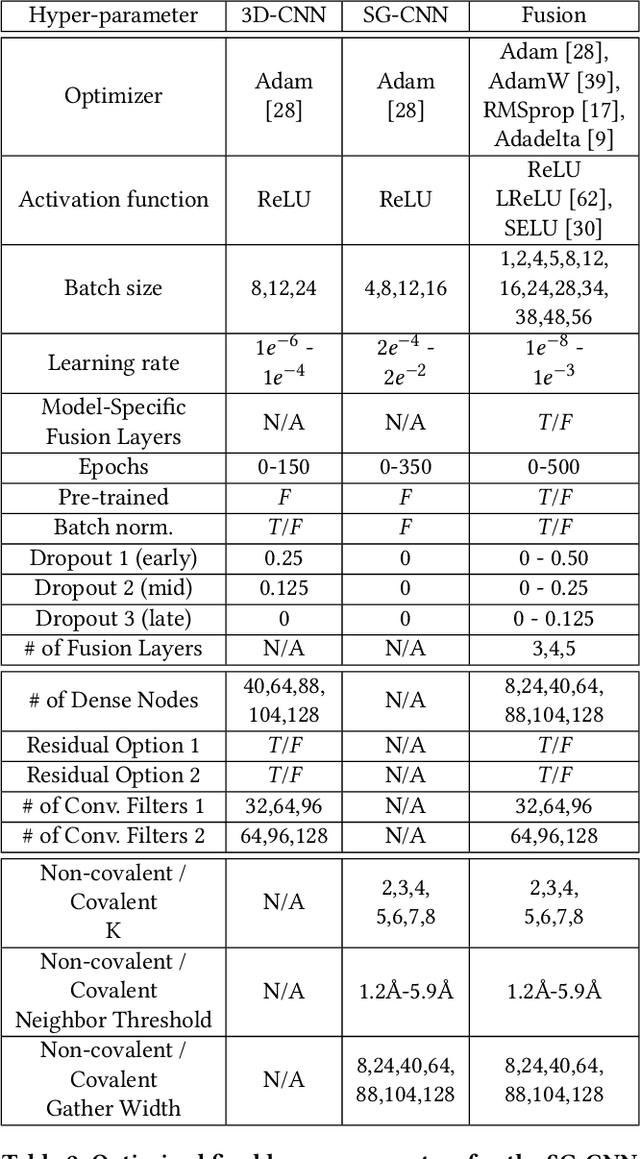
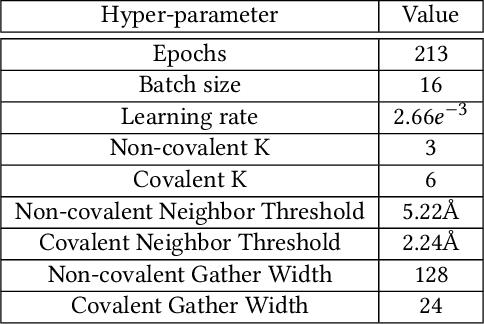
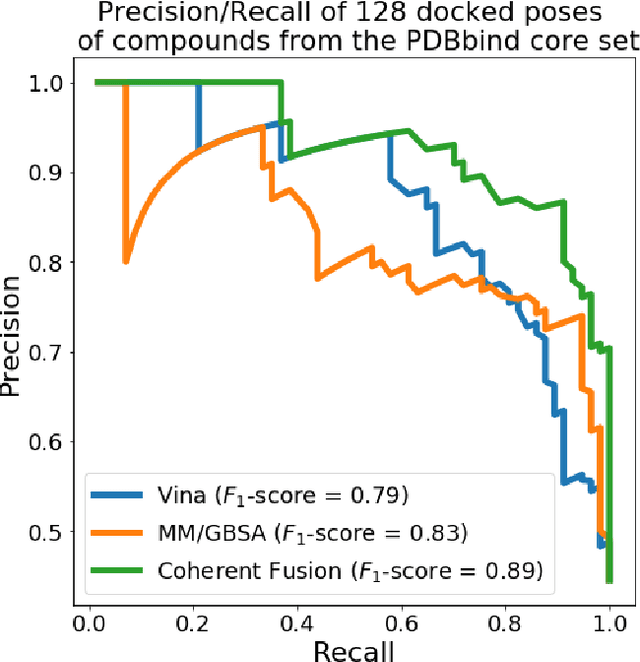
Abstract:Structure-based Deep Fusion models were recently shown to outperform several physics- and machine learning-based protein-ligand binding affinity prediction methods. As part of a multi-institutional COVID-19 pandemic response, over 500 million small molecules were computationally screened against four protein structures from the novel coronavirus (SARS-CoV-2), which causes COVID-19. Three enhancements to Deep Fusion were made in order to evaluate more than 5 billion docked poses on SARS-CoV-2 protein targets. First, the Deep Fusion concept was refined by formulating the architecture as one, coherently backpropagated model (Coherent Fusion) to improve binding-affinity prediction accuracy. Secondly, the model was trained using a distributed, genetic hyper-parameter optimization. Finally, a scalable, high-throughput screening capability was developed to maximize the number of ligands evaluated and expedite the path to experimental evaluation. In this work, we present both the methods developed for machine learning-based high-throughput screening and results from using our computational pipeline to find SARS-CoV-2 inhibitors.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge