Haomin Chen
INTRPRT: A Systematic Review of and Guidelines for Designing and Validating Transparent AI in Medical Image Analysis
Dec 21, 2021
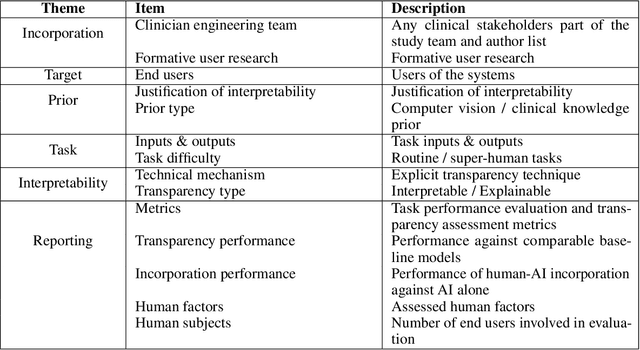

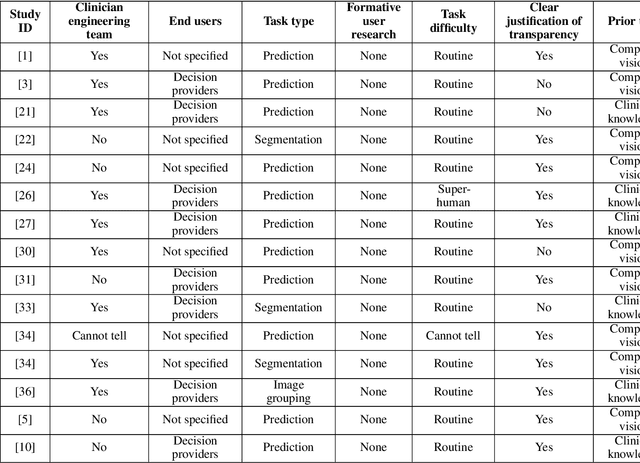
Abstract:Transparency in Machine Learning (ML), attempts to reveal the working mechanisms of complex models. Transparent ML promises to advance human factors engineering goals of human-centered AI in the target users. From a human-centered design perspective, transparency is not a property of the ML model but an affordance, i.e. a relationship between algorithm and user; as a result, iterative prototyping and evaluation with users is critical to attaining adequate solutions that afford transparency. However, following human-centered design principles in healthcare and medical image analysis is challenging due to the limited availability of and access to end users. To investigate the state of transparent ML in medical image analysis, we conducted a systematic review of the literature. Our review reveals multiple severe shortcomings in the design and validation of transparent ML for medical image analysis applications. We find that most studies to date approach transparency as a property of the model itself, similar to task performance, without considering end users during neither development nor evaluation. Additionally, the lack of user research, and the sporadic validation of transparency claims put contemporary research on transparent ML for medical image analysis at risk of being incomprehensible to users, and thus, clinically irrelevant. To alleviate these shortcomings in forthcoming research while acknowledging the challenges of human-centered design in healthcare, we introduce the INTRPRT guideline, a systematic design directive for transparent ML systems in medical image analysis. The INTRPRT guideline suggests formative user research as the first step of transparent model design to understand user needs and domain requirements. Following this process produces evidence to support design choices, and ultimately, increases the likelihood that the algorithms afford transparency.
An Interpretable Algorithm for Uveal Melanoma Subtyping from Whole Slide Cytology Images
Aug 13, 2021



Abstract:Algorithmic decision support is rapidly becoming a staple of personalized medicine, especially for high-stakes recommendations in which access to certain information can drastically alter the course of treatment, and thus, patient outcome; a prominent example is radiomics for cancer subtyping. Because in these scenarios the stakes are high, it is desirable for decision systems to not only provide recommendations but supply transparent reasoning in support thereof. For learning-based systems, this can be achieved through an interpretable design of the inference pipeline. Herein we describe an automated yet interpretable system for uveal melanoma subtyping with digital cytology images from fine needle aspiration biopsies. Our method embeds every automatically segmented cell of a candidate cytology image as a point in a 2D manifold defined by many representative slides, which enables reasoning about the cell-level composition of the tissue sample, paving the way for interpretable subtyping of the biopsy. Finally, a rule-based slide-level classification algorithm is trained on the partitions of the circularly distorted 2D manifold. This process results in a simple rule set that is evaluated automatically but highly transparent for human verification. On our in house cytology dataset of 88 uveal melanoma patients, the proposed method achieves an accuracy of 87.5% that compares favorably to all competing approaches, including deep "black box" models. The method comes with a user interface to facilitate interaction with cell-level content, which may offer additional insights for pathological assessment.
Deep Hiearchical Multi-Label Classification Applied to Chest X-Ray Abnormality Taxonomies
Sep 23, 2020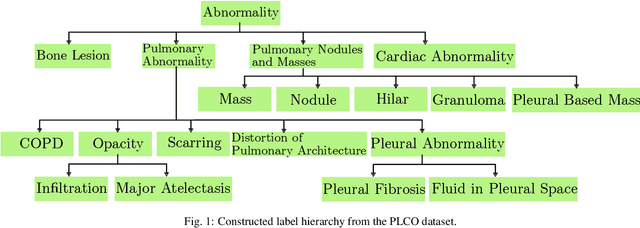
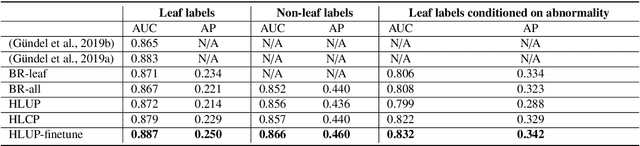
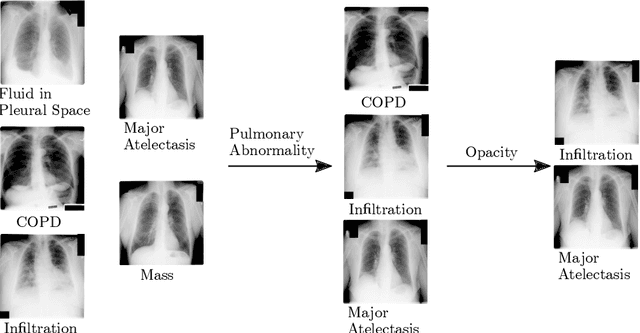
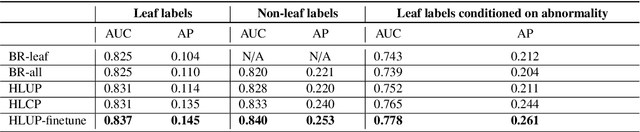
Abstract:CXRs are a crucial and extraordinarily common diagnostic tool, leading to heavy research for CAD solutions. However, both high classification accuracy and meaningful model predictions that respect and incorporate clinical taxonomies are crucial for CAD usability. To this end, we present a deep HMLC approach for CXR CAD. Different than other hierarchical systems, we show that first training the network to model conditional probability directly and then refining it with unconditional probabilities is key in boosting performance. In addition, we also formulate a numerically stable cross-entropy loss function for unconditional probabilities that provides concrete performance improvements. Finally, we demonstrate that HMLC can be an effective means to manage missing or incomplete labels. To the best of our knowledge, we are the first to apply HMLC to medical imaging CAD. We extensively evaluate our approach on detecting abnormality labels from the CXR arm of the PLCO dataset, which comprises over $198,000$ manually annotated CXRs. When using complete labels, we report a mean AUC of 0.887, the highest yet reported for this dataset. These results are supported by ancillary experiments on the PadChest dataset, where we also report significant improvements, 1.2% and 4.1% in AUC and AP, respectively over strong "flat" classifiers. Finally, we demonstrate that our HMLC approach can much better handle incompletely labelled data. These performance improvements, combined with the inherent usefulness of taxonomic predictions, indicate that our approach represents a useful step forward for CXR CAD.
Anatomy-Aware Siamese Network: Exploiting Semantic Asymmetry for Accurate Pelvic Fracture Detection in X-ray Images
Jul 12, 2020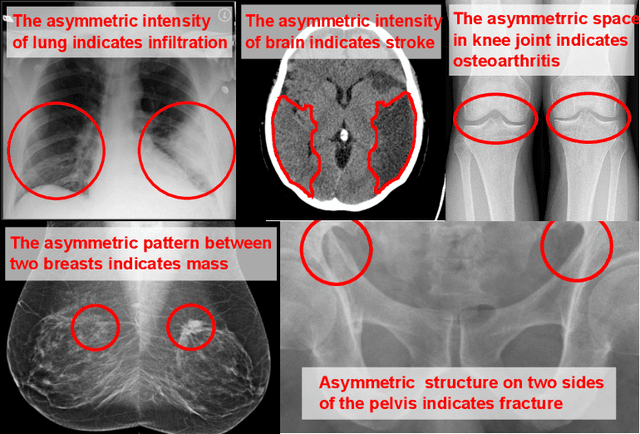
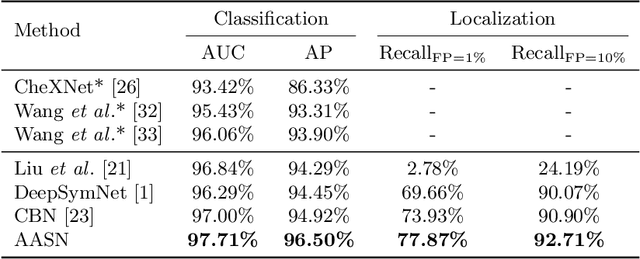
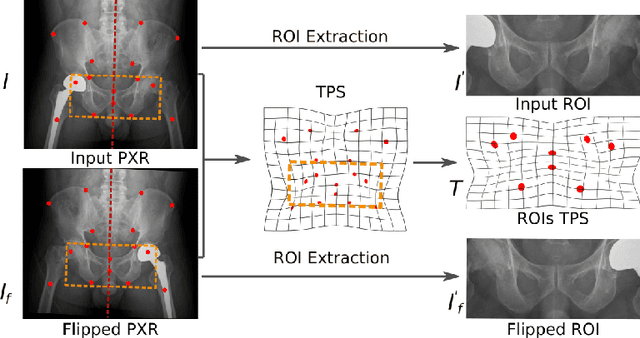
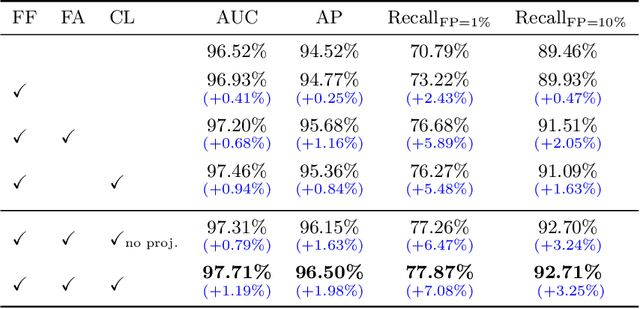
Abstract:Visual cues of enforcing bilaterally symmetric anatomies as normal findings are widely used in clinical practice to disambiguate subtle abnormalities from medical images. So far, inadequate research attention has been received on effectively emulating this practice in CAD methods. In this work, we exploit semantic anatomical symmetry or asymmetry analysis in a complex CAD scenario, i.e., anterior pelvic fracture detection in trauma PXRs, where semantically pathological (refer to as fracture) and non-pathological (e.g., pose) asymmetries both occur. Visually subtle yet pathologically critical fracture sites can be missed even by experienced clinicians, when limited diagnosis time is permitted in emergency care. We propose a novel fracture detection framework that builds upon a Siamese network enhanced with a spatial transformer layer to holistically analyze symmetric image features. Image features are spatially formatted to encode bilaterally symmetric anatomies. A new contrastive feature learning component in our Siamese network is designed to optimize the deep image features being more salient corresponding to the underlying semantic asymmetries (caused by pelvic fracture occurrences). Our proposed method have been extensively evaluated on 2,359 PXRs from unique patients (the largest study to-date), and report an area under ROC curve score of 0.9771. This is the highest among state-of-the-art fracture detection methods, with improved clinical indications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge