Guanghua Xiao
MEDVISTAGYM: A Scalable Training Environment for Thinking with Medical Images via Tool-Integrated Reinforcement Learning
Jan 12, 2026Abstract:Vision language models (VLMs) achieve strong performance on general image understanding but struggle to think with medical images, especially when performing multi-step reasoning through iterative visual interaction. Medical VLMs often rely on static visual embeddings and single-pass inference, preventing models from re-examining, verifying, or refining visual evidence during reasoning. While tool-integrated reasoning offers a promising path forward, open-source VLMs lack the training infrastructure to learn effective tool selection, invocation, and coordination in multi-modal medical reasoning. We introduce MedVistaGym, a scalable and interactive training environment that incentivizes tool-integrated visual reasoning for medical image analysis. MedVistaGym equips VLMs to determine when and which tools to invoke, localize task-relevant image regions, and integrate single or multiple sub-image evidence into interleaved multimodal reasoning within a unified, executable interface for agentic training. Using MedVistaGym, we train MedVistaGym-R1 to interleave tool use with agentic reasoning through trajectory sampling and end-to-end reinforcement learning. Across six medical VQA benchmarks, MedVistaGym-R1-8B exceeds comparably sized tool-augmented baselines by 19.10% to 24.21%, demonstrating that structured agentic training--not tool access alone--unlocks effective tool-integrated reasoning for medical image analysis.
MedAgentGym: Training LLM Agents for Code-Based Medical Reasoning at Scale
Jun 04, 2025



Abstract:We introduce MedAgentGYM, the first publicly available training environment designed to enhance coding-based medical reasoning capabilities in large language model (LLM) agents. MedAgentGYM comprises 72,413 task instances across 129 categories derived from authentic real-world biomedical scenarios. Tasks are encapsulated within executable coding environments, each featuring detailed task descriptions, interactive feedback mechanisms, verifiable ground-truth annotations, and scalable training trajectory generation. Extensive benchmarking of over 30 LLMs reveals a notable performance disparity between commercial API-based models and open-source counterparts. Leveraging MedAgentGYM, Med-Copilot-7B achieves substantial performance gains through supervised fine-tuning (+36.44%) and continued reinforcement learning (+42.47%), emerging as an affordable and privacy-preserving alternative competitive with gpt-4o. By offering both a comprehensive benchmark and accessible, expandable training resources within unified execution environments, MedAgentGYM delivers an integrated platform to develop LLM-based coding assistants for advanced biomedical research and practice.
Large language models enabled multiagent ensemble method for efficient EHR data labeling
Oct 21, 2024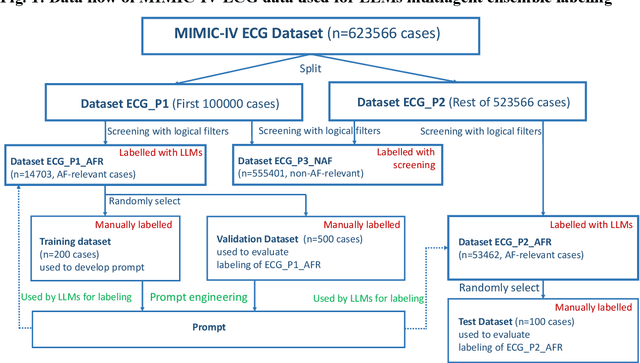
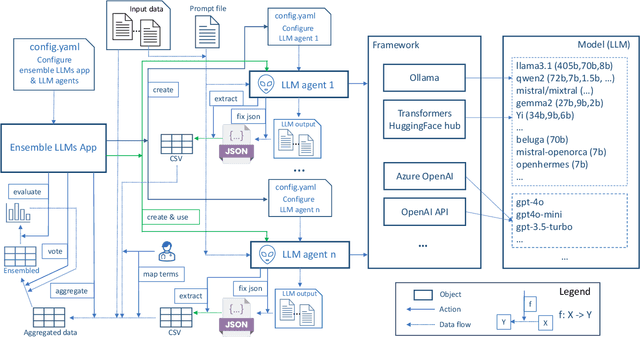
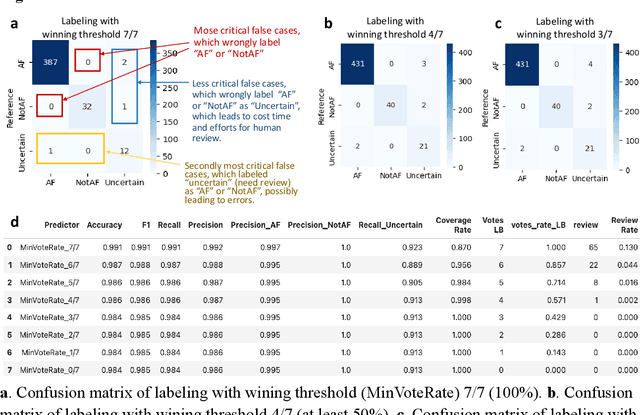
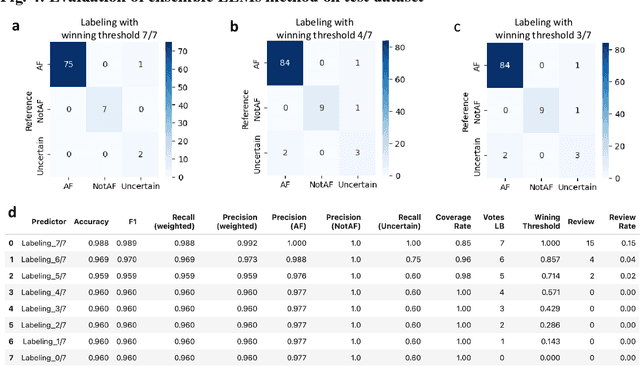
Abstract:This study introduces a novel multiagent ensemble method powered by LLMs to address a key challenge in ML - data labeling, particularly in large-scale EHR datasets. Manual labeling of such datasets requires domain expertise and is labor-intensive, time-consuming, expensive, and error-prone. To overcome this bottleneck, we developed an ensemble LLMs method and demonstrated its effectiveness in two real-world tasks: (1) labeling a large-scale unlabeled ECG dataset in MIMIC-IV; (2) identifying social determinants of health (SDOH) from the clinical notes of EHR. Trading off benefits and cost, we selected a pool of diverse open source LLMs with satisfactory performance. We treat each LLM's prediction as a vote and apply a mechanism of majority voting with minimal winning threshold for ensemble. We implemented an ensemble LLMs application for EHR data labeling tasks. By using the ensemble LLMs and natural language processing, we labeled MIMIC-IV ECG dataset of 623,566 ECG reports with an estimated accuracy of 98.2%. We applied the ensemble LLMs method to identify SDOH from social history sections of 1,405 EHR clinical notes, also achieving competitive performance. Our experiments show that the ensemble LLMs can outperform individual LLM even the best commercial one, and the method reduces hallucination errors. From the research, we found that (1) the ensemble LLMs method significantly reduces the time and effort required for labeling large-scale EHR data, automating the process with high accuracy and quality; (2) the method generalizes well to other text data labeling tasks, as shown by its application to SDOH identification; (3) the ensemble of a group of diverse LLMs can outperform or match the performance of the best individual LLM; and (4) the ensemble method substantially reduces hallucination errors. This approach provides a scalable and efficient solution to data-labeling challenges.
Discovering Clinically Meaningful Shape Features for the Analysis of Tumor Pathology Images
Dec 09, 2020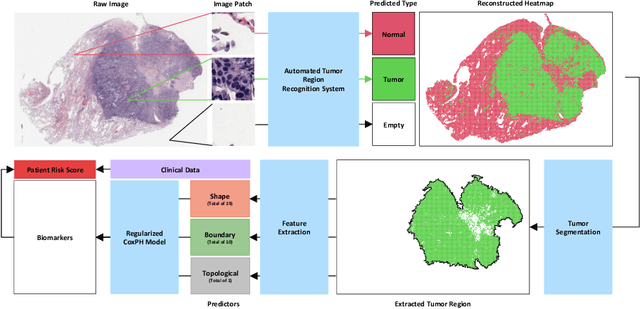
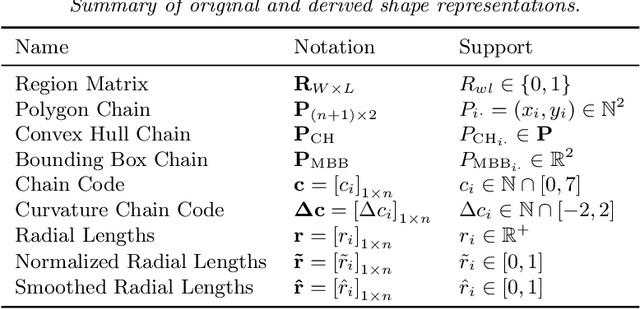
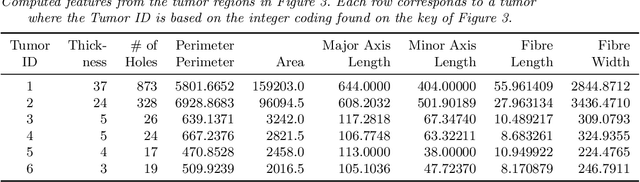
Abstract:With the advanced imaging technology, digital pathology imaging of tumor tissue slides is becoming a routine clinical procedure for cancer diagnosis. This process produces massive imaging data that capture histological details in high resolution. Recent developments in deep-learning methods have enabled us to automatically detect and characterize the tumor regions in pathology images at large scale. From each identified tumor region, we extracted 30 well-defined descriptors that quantify its shape, geometry, and topology. We demonstrated how those descriptor features were associated with patient survival outcome in lung adenocarcinoma patients from the National Lung Screening Trial (n=143). Besides, a descriptor-based prognostic model was developed and validated in an independent patient cohort from The Cancer Genome Atlas Program program (n=318). This study proposes new insights into the relationship between tumor shape, geometrical, and topological features and patient prognosis. We provide software in the form of R code on GitHub: https://github.com/estfernandez/Slide_Image_Segmentation_and_Extraction.
Predicting survival outcomes using topological features of tumor pathology images
Dec 07, 2020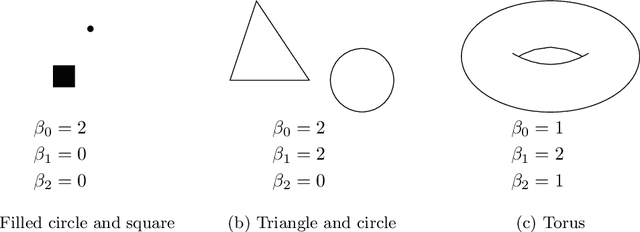
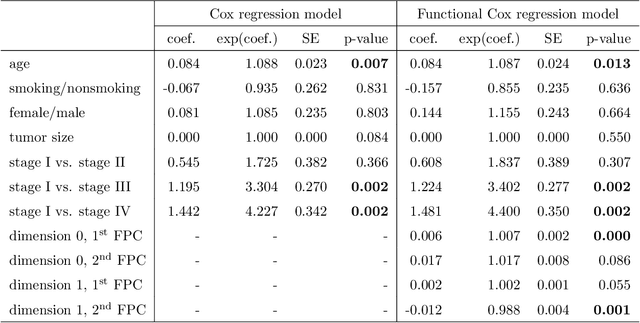
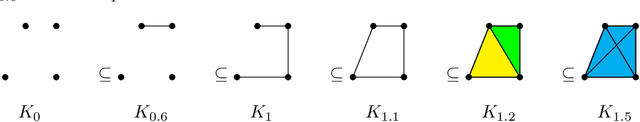
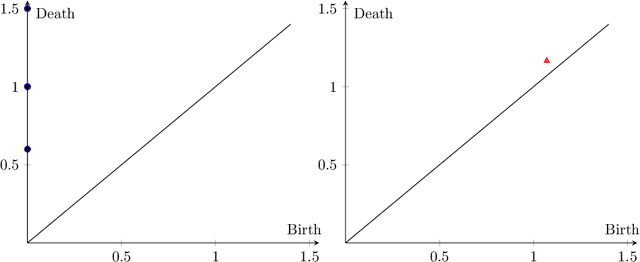
Abstract:Tumor shape and size have been used as important markers for cancer diagnosis and treatment. Recent developments in medical imaging technology enable more detailed segmentation of tumor regions in high resolution. This paper proposes a topological feature to characterize tumor progression from digital pathology images and examine its effect on the time-to-event data. We develop distance transform for pathology images and show that a topological summary statistic computed by persistent homology quantifies tumor shape, size, distribution, and connectivity. The topological features are represented in functional space and used as functional predictors in a functional Cox regression model. A case study is conducted using non-small cell lung cancer pathology images. The results show that the topological features predict survival prognosis after adjusting for age, sex, smoking status, stage, and size of tumors. Also, the topological features with non-zero effects correspond to the shapes that are known to be related to tumor progression. Our study provides a new perspective for understanding tumor shape and patient prognosis.
ConvPath: A Software Tool for Lung Adenocarcinoma Digital Pathological Image Analysis Aided by Convolutional Neural Network
Sep 20, 2018



Abstract:The spatial distributions of different types of cells could reveal a cancer cell growth pattern, its relationships with the tumor microenvironment and the immune response of the body, all of which represent key hallmarks of cancer. However, manually recognizing and localizing all the cells in pathology slides are almost impossible. In this study, we developed an automated cell type classification pipeline, ConvPath, which includes nuclei segmentation, convolutional neural network-based tumor, stromal and lymphocytes classification, and extraction of tumor microenvironment related features for lung cancer pathology images. The overall classification accuracy is 92.9% and 90.1% in training and independent testing datasets, respectively. By identifying cells and classifying cell types, this pipeline can convert a pathology image into a spatial map of tumor, stromal and lymphocyte cells. From this spatial map, we can extracted features that characterize the tumor micro-environment. Based on these features, we developed an image feature-based prognostic model and validated the model in two independent cohorts. The predicted risk group serves as an independent prognostic factor, after adjusting for clinical variables that include age, gender, smoking status, and stage.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge