Georgiana Neag
Reference-Free Multi-Modality Volume Registration of X-Ray Microscopy and Light-Sheet Fluorescence Microscopy
Apr 23, 2024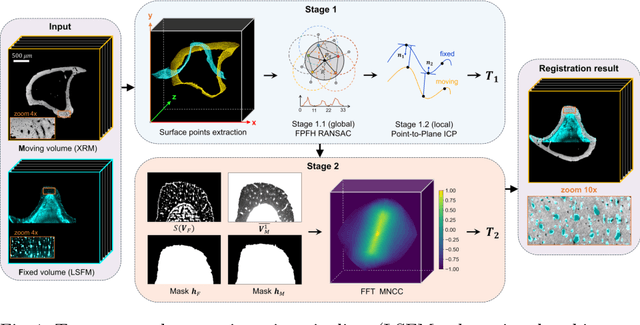
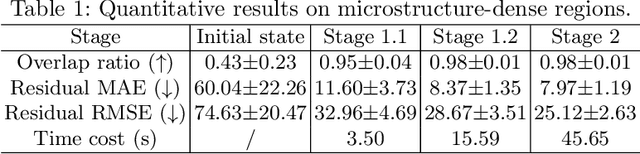
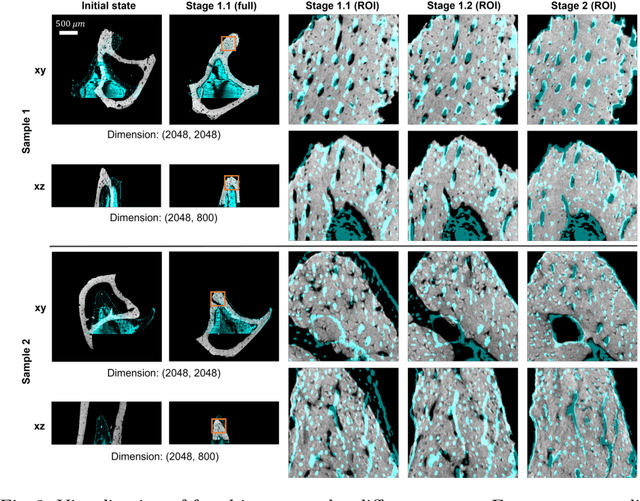
Abstract:Recently, X-ray microscopy (XRM) and light-sheet fluorescence microscopy (LSFM) have emerged as two pivotal imaging tools in preclinical research on bone remodeling diseases, offering micrometer-level resolution. Integrating these complementary modalities provides a holistic view of bone microstructures, facilitating function-oriented volume analysis across different disease cycles. However, registering such independently acquired large-scale volumes is extremely challenging under real and reference-free scenarios. This paper presents a fast two-stage pipeline for volume registration of XRM and LSFM. The first stage extracts the surface features and employs two successive point cloud-based methods for coarse alignment. The second stage fine-tunes the initial alignment using a modified cross-correlation method, ensuring precise volumetric registration. Moreover, we propose residual similarity as a novel metric to assess the alignment of two complementary modalities. The results imply robust gradual improvement across the stages. In the end, all correlating microstructures, particularly lacunae in XRM and bone cells in LSFM, are precisely matched, enabling new insights into bone diseases like osteoporosis which are a substantial burden in aging societies.
Motion Compensation via Epipolar Consistency for In-Vivo X-Ray Microscopy
Mar 01, 2023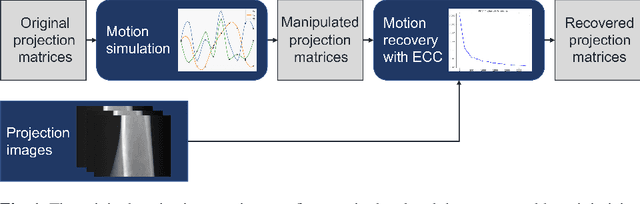
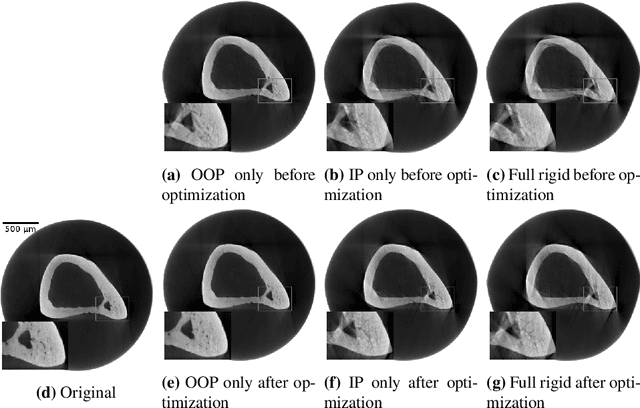
Abstract:Intravital X-ray microscopy (XRM) in preclinical mouse models is of vital importance for the identification of microscopic structural pathological changes in the bone which are characteristic of osteoporosis. The complexity of this method stems from the requirement for high-quality 3D reconstructions of the murine bones. However, respiratory motion and muscle relaxation lead to inconsistencies in the projection data which result in artifacts in uncompensated reconstructions. Motion compensation using epipolar consistency conditions (ECC) has previously shown good performance in clinical CT settings. Here, we explore whether such algorithms are suitable for correcting motion-corrupted XRM data. Different rigid motion patterns are simulated and the quality of the motion-compensated reconstructions is assessed. The method is able to restore microscopic features for out-of-plane motion, but artifacts remain for more realistic motion patterns including all six degrees of freedom of rigid motion. Therefore, ECC is valuable for the initial alignment of the projection data followed by further fine-tuning of motion parameters using a reconstruction-based method
Noise2Contrast: Multi-Contrast Fusion Enables Self-Supervised Tomographic Image Denoising
Dec 09, 2022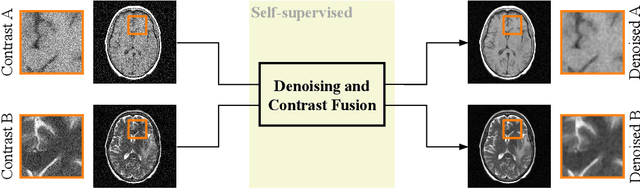
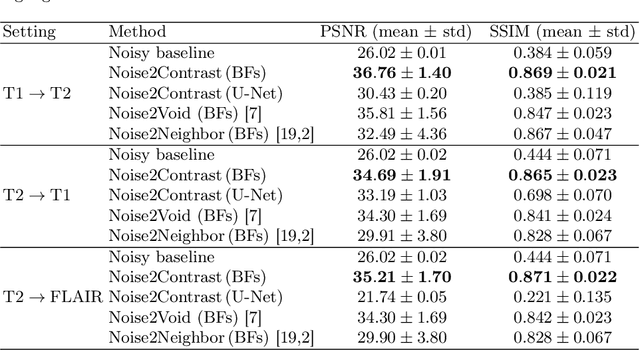
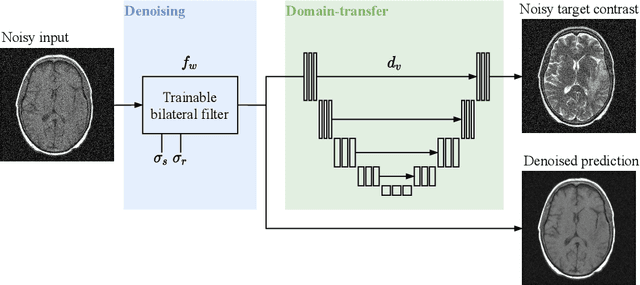
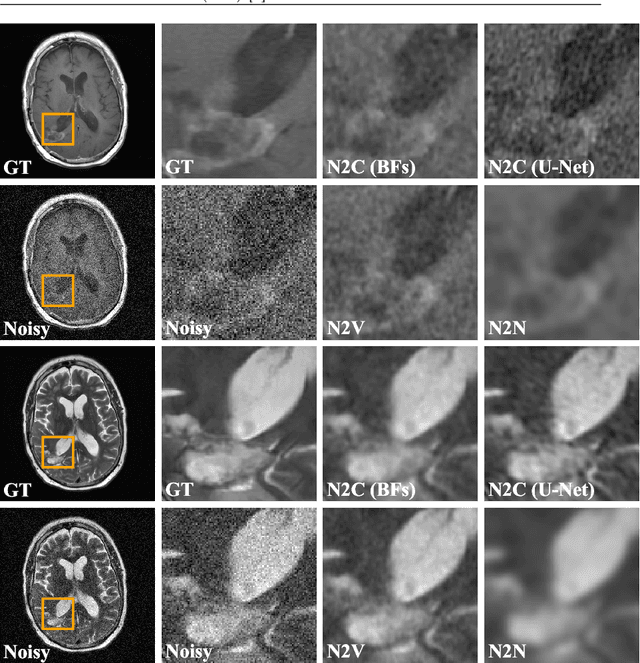
Abstract:Self-supervised image denoising techniques emerged as convenient methods that allow training denoising models without requiring ground-truth noise-free data. Existing methods usually optimize loss metrics that are calculated from multiple noisy realizations of similar images, e.g., from neighboring tomographic slices. However, those approaches fail to utilize the multiple contrasts that are routinely acquired in medical imaging modalities like MRI or dual-energy CT. In this work, we propose the new self-supervised training scheme Noise2Contrast that combines information from multiple measured image contrasts to train a denoising model. We stack denoising with domain-transfer operators to utilize the independent noise realizations of different image contrasts to derive a self-supervised loss. The trained denoising operator achieves convincing quantitative and qualitative results, outperforming state-of-the-art self-supervised methods by 4.7-11.0%/4.8-7.3% (PSNR/SSIM) on brain MRI data and by 43.6-50.5%/57.1-77.1% (PSNR/SSIM) on dual-energy CT X-ray microscopy data with respect to the noisy baseline. Our experiments on different real measured data sets indicate that Noise2Contrast training generalizes to other multi-contrast imaging modalities.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge