Georgia Doumou
Explainable Shape Analysis through Deep Hierarchical Generative Models: Application to Cardiac Remodeling
Jun 28, 2019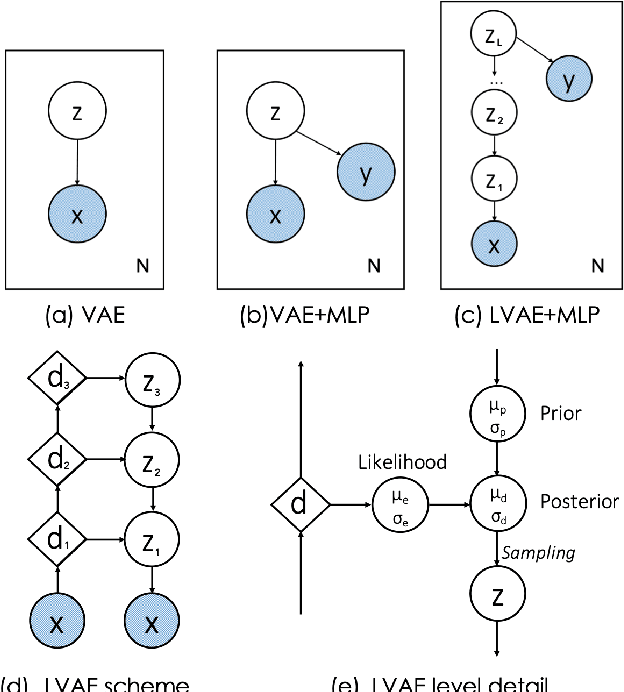
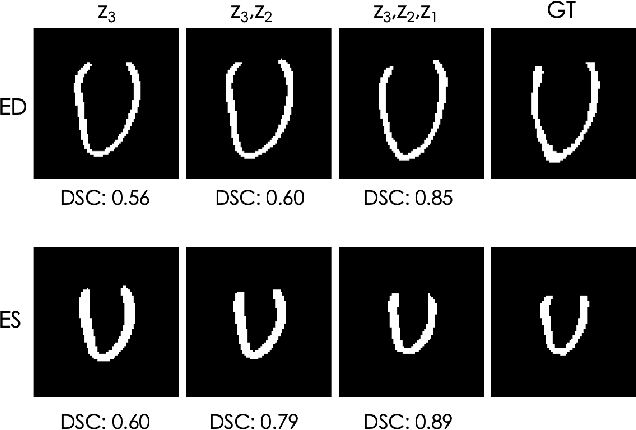
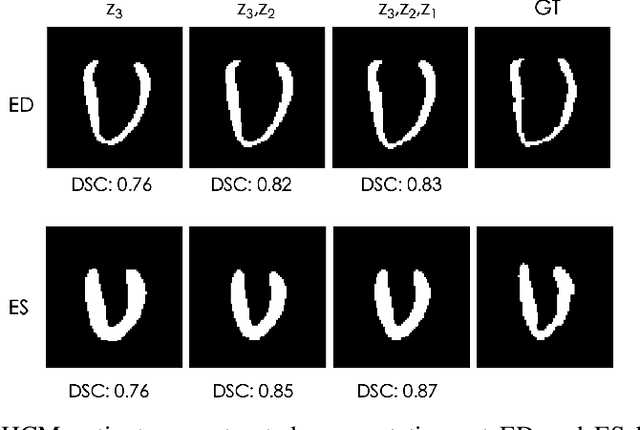
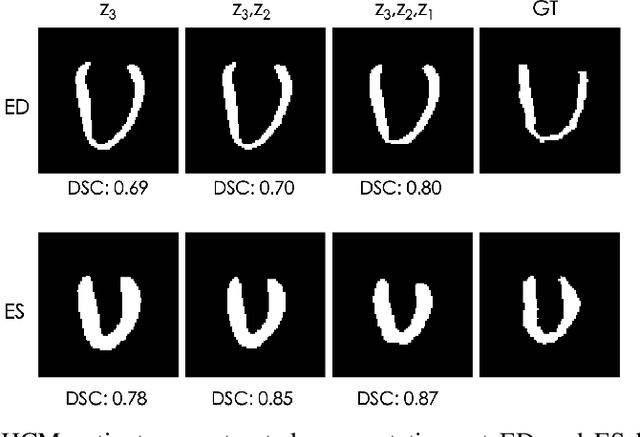
Abstract:Quantification of anatomical shape changes still relies on scalar global indexes which are largely insensitive to regional or asymmetric modifications. Accurate assessment of pathology-driven anatomical remodeling is a crucial step for the diagnosis and treatment of heart conditions. Deep learning approaches have recently achieved wide success in the analysis of medical images, but they lack interpretability in the feature extraction and decision processes. In this work, we propose a new interpretable deep learning model for shape analysis. In particular, we exploit deep generative networks to model a population of anatomical segmentations through a hierarchy of conditional latent variables. At the highest level of this hierarchy, a two-dimensional latent space is simultaneously optimised to discriminate distinct clinical conditions, enabling the direct visualisation of the classification space. Moreover, the anatomical variability encoded by this discriminative latent space can be visualised in the segmentation space thanks to the generative properties of the model, making the classification task transparent. This approach yielded high accuracy in the categorisation of healthy and remodelled hearts when tested on unseen segmentations from our own multi-centre dataset as well as in an external validation set. More importantly, it enabled the visualisation in three-dimensions of the most discriminative anatomical features between the two conditions. The proposed approach scales effectively to large populations, facilitating high-throughput analysis of normal anatomy and pathology in large-scale studies of volumetric imaging.
Automatic 3D bi-ventricular segmentation of cardiac images by a shape-constrained multi-task deep learning approach
Aug 28, 2018



Abstract:Deep learning approaches have achieved state-of-the-art performance in cardiac magnetic resonance (CMR) image segmentation. However, most approaches have focused on learning image intensity features for segmentation, whereas the incorporation of anatomical shape priors has received less attention. In this paper, we combine a multi-task deep learning approach with atlas propagation to develop a shape-constrained bi-ventricular segmentation pipeline for short-axis CMR volumetric images. The pipeline first employs a fully convolutional network (FCN) that learns segmentation and landmark localisation tasks simultaneously. The architecture of the proposed FCN uses a 2.5D representation, thus combining the computational advantage of 2D FCNs networks and the capability of addressing 3D spatial consistency without compromising segmentation accuracy. Moreover, the refinement step is designed to explicitly enforce a shape constraint and improve segmentation quality. This step is effective for overcoming image artefacts (e.g. due to different breath-hold positions and large slice thickness), which preclude the creation of anatomically meaningful 3D cardiac shapes. The proposed pipeline is fully automated, due to network's ability to infer landmarks, which are then used downstream in the pipeline to initialise atlas propagation. We validate the pipeline on 1831 healthy subjects and 649 subjects with pulmonary hypertension. Extensive numerical experiments on the two datasets demonstrate that our proposed method is robust and capable of producing accurate, high-resolution and anatomically smooth bi-ventricular 3D models, despite the artefacts in input CMR volumes.
Deep nested level sets: Fully automated segmentation of cardiac MR images in patients with pulmonary hypertension
Jul 27, 2018


Abstract:In this paper we introduce a novel and accurate optimisation method for segmentation of cardiac MR (CMR) images in patients with pulmonary hypertension (PH). The proposed method explicitly takes into account the image features learned from a deep neural network. To this end, we estimate simultaneous probability maps over region and edge locations in CMR images using a fully convolutional network. Due to the distinct morphology of the heart in patients with PH, these probability maps can then be incorporated in a single nested level set optimisation framework to achieve multi-region segmentation with high efficiency. The proposed method uses an automatic way for level set initialisation and thus the whole optimisation is fully automated. We demonstrate that the proposed deep nested level set (DNLS) method outperforms existing state-of-the-art methods for CMR segmentation in PH patients.
Learning Interpretable Anatomical Features Through Deep Generative Models: Application to Cardiac Remodeling
Jul 18, 2018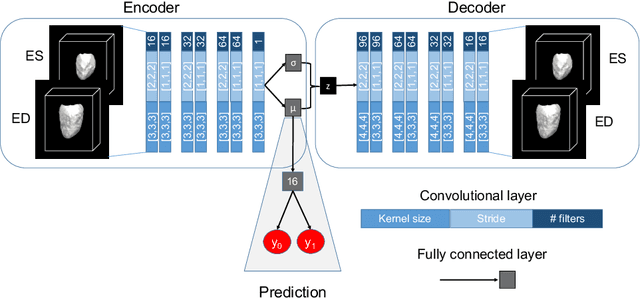
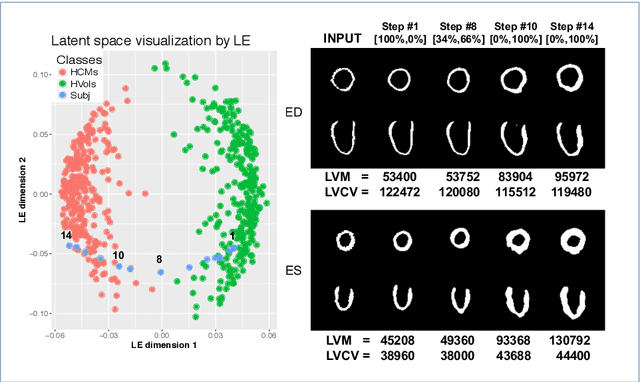
Abstract:Alterations in the geometry and function of the heart define well-established causes of cardiovascular disease. However, current approaches to the diagnosis of cardiovascular diseases often rely on subjective human assessment as well as manual analysis of medical images. Both factors limit the sensitivity in quantifying complex structural and functional phenotypes. Deep learning approaches have recently achieved success for tasks such as classification or segmentation of medical images, but lack interpretability in the feature extraction and decision processes, limiting their value in clinical diagnosis. In this work, we propose a 3D convolutional generative model for automatic classification of images from patients with cardiac diseases associated with structural remodeling. The model leverages interpretable task-specific anatomic patterns learned from 3D segmentations. It further allows to visualise and quantify the learned pathology-specific remodeling patterns in the original input space of the images. This approach yields high accuracy in the categorization of healthy and hypertrophic cardiomyopathy subjects when tested on unseen MR images from our own multi-centre dataset (100%) as well on the ACDC MICCAI 2017 dataset (90%). We believe that the proposed deep learning approach is a promising step towards the development of interpretable classifiers for the medical imaging domain, which may help clinicians to improve diagnostic accuracy and enhance patient risk-stratification.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge