Daniel Borders
PLUTO: Pathology-Universal Transformer
May 13, 2024



Abstract:Pathology is the study of microscopic inspection of tissue, and a pathology diagnosis is often the medical gold standard to diagnose disease. Pathology images provide a unique challenge for computer-vision-based analysis: a single pathology Whole Slide Image (WSI) is gigapixel-sized and often contains hundreds of thousands to millions of objects of interest across multiple resolutions. In this work, we propose PathoLogy Universal TransfOrmer (PLUTO): a light-weight pathology FM that is pre-trained on a diverse dataset of 195 million image tiles collected from multiple sites and extracts meaningful representations across multiple WSI scales that enable a large variety of downstream pathology tasks. In particular, we design task-specific adaptation heads that utilize PLUTO's output embeddings for tasks which span pathology scales ranging from subcellular to slide-scale, including instance segmentation, tile classification, and slide-level prediction. We compare PLUTO's performance to other state-of-the-art methods on a diverse set of external and internal benchmarks covering multiple biologically relevant tasks, tissue types, resolutions, stains, and scanners. We find that PLUTO matches or outperforms existing task-specific baselines and pathology-specific foundation models, some of which use orders-of-magnitude larger datasets and model sizes when compared to PLUTO. Our findings present a path towards a universal embedding to power pathology image analysis, and motivate further exploration around pathology foundation models in terms of data diversity, architectural improvements, sample efficiency, and practical deployability in real-world applications.
Improved statistical benchmarking of digital pathology models using pairwise frames evaluation
Jun 07, 2023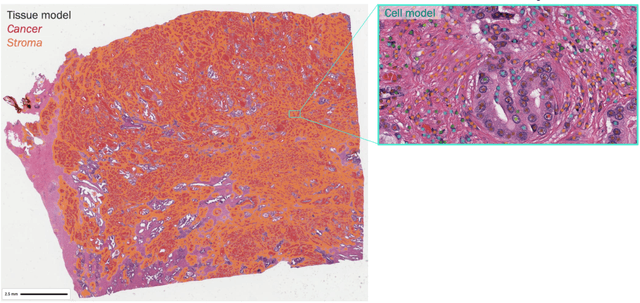
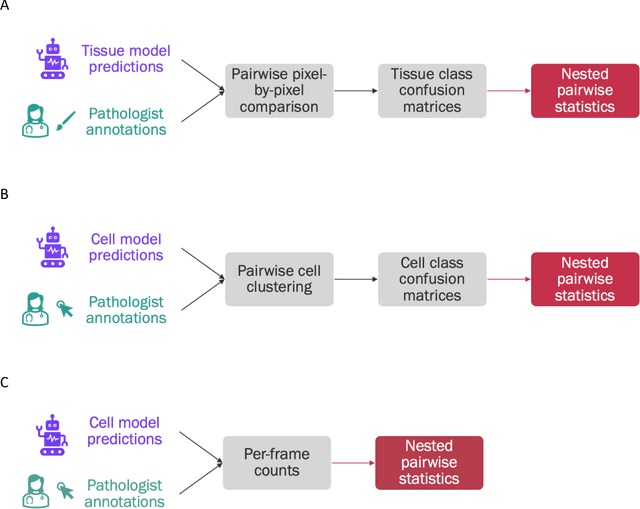
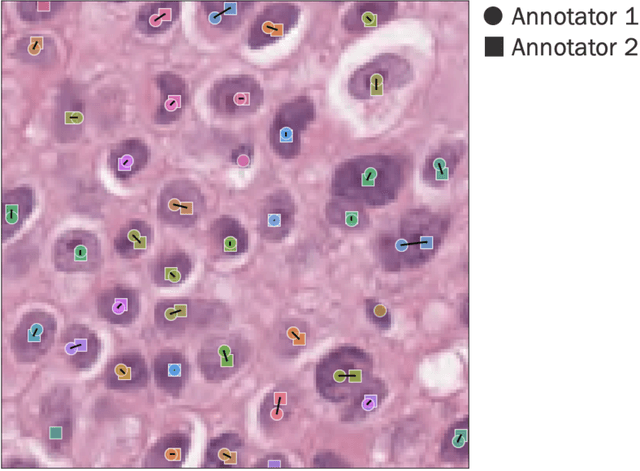
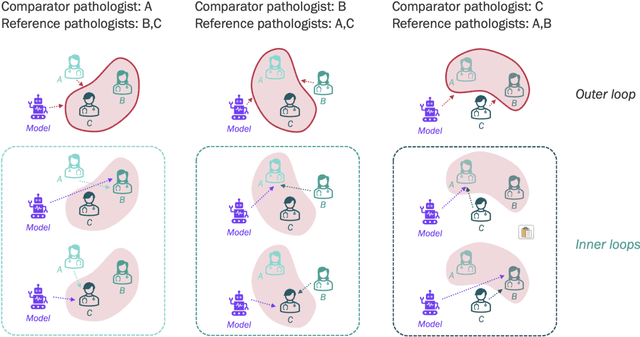
Abstract:Nested pairwise frames is a method for relative benchmarking of cell or tissue digital pathology models against manual pathologist annotations on a set of sampled patches. At a high level, the method compares agreement between a candidate model and pathologist annotations with agreement among pathologists' annotations. This evaluation framework addresses fundamental issues of data size and annotator variability in using manual pathologist annotations as a source of ground truth for model validation. We implemented nested pairwise frames evaluation for tissue classification, cell classification, and cell count prediction tasks and show results for cell and tissue models deployed on an H&E-stained melanoma dataset.
Deep Adversarial Training for Multi-Organ Nuclei Segmentation in Histopathology Images
Oct 19, 2018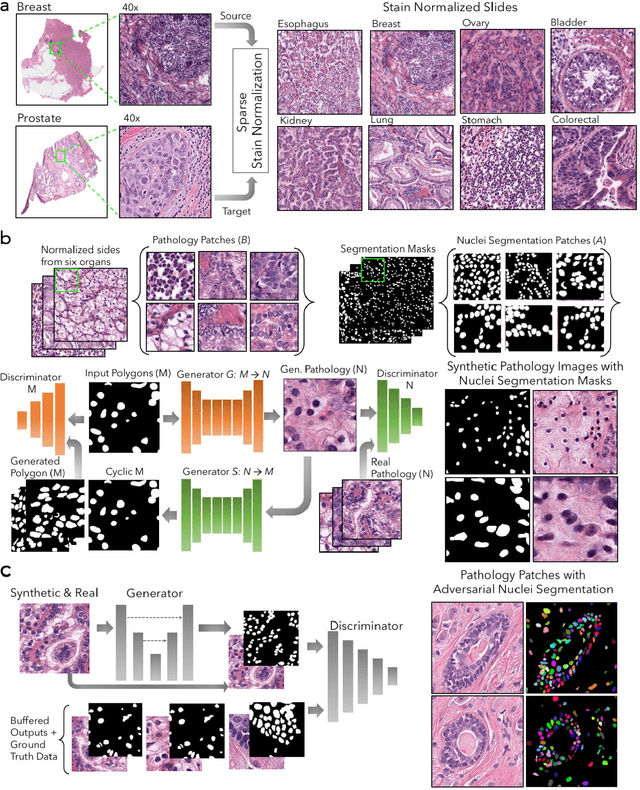
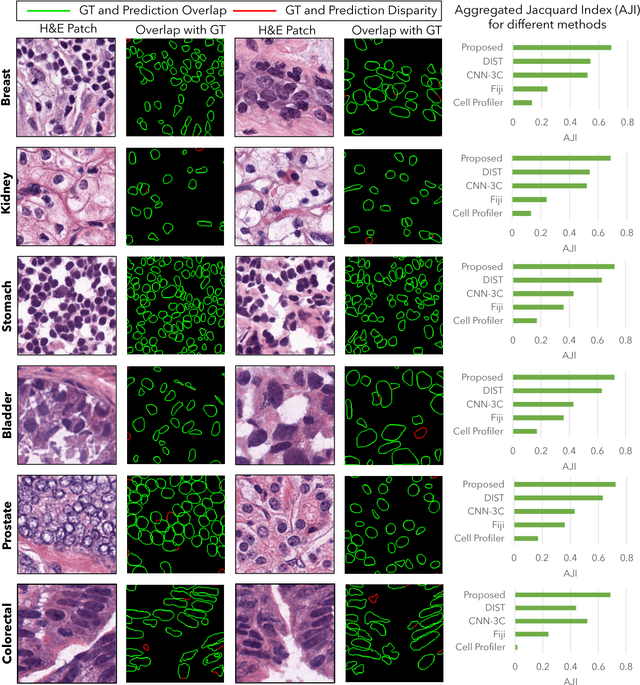
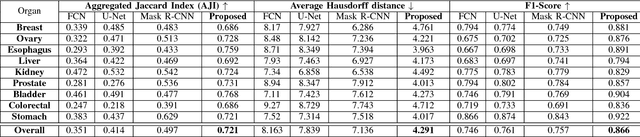
Abstract:Nuclei segmentation is a fundamental task that is critical for various computational pathology applications including nuclei morphology analysis, cell type classification, and cancer grading. Conventional vision-based methods for nuclei segmentation struggle in challenging cases and deep learning approaches have proven to be more robust and generalizable. However, CNNs require large amounts of labeled histopathology data. Moreover, conventional CNN-based approaches lack structured prediction capabilities which are required to distinguish overlapping and clumped nuclei. Here, we present an approach to nuclei segmentation that overcomes these challenges by utilizing a conditional generative adversarial network (cGAN) trained with synthetic and real data. We generate a large dataset of H&E training images with perfect nuclei segmentation labels using an unpaired GAN framework. This synthetic data along with real histopathology data from six different organs are used to train a conditional GAN with spectral normalization and gradient penalty for nuclei segmentation. This adversarial regression framework enforces higher order consistency when compared to conventional CNN models. We demonstrate that this nuclei segmentation approach generalizes across different organs, sites, patients and disease states, and outperforms conventional approaches, especially in isolating individual and overlapping nuclei.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge