Chen-Chen Fan
A Joint Representation Using Continuous and Discrete Features for Cardiovascular Diseases Risk Prediction on Chest CT Scans
Oct 24, 2024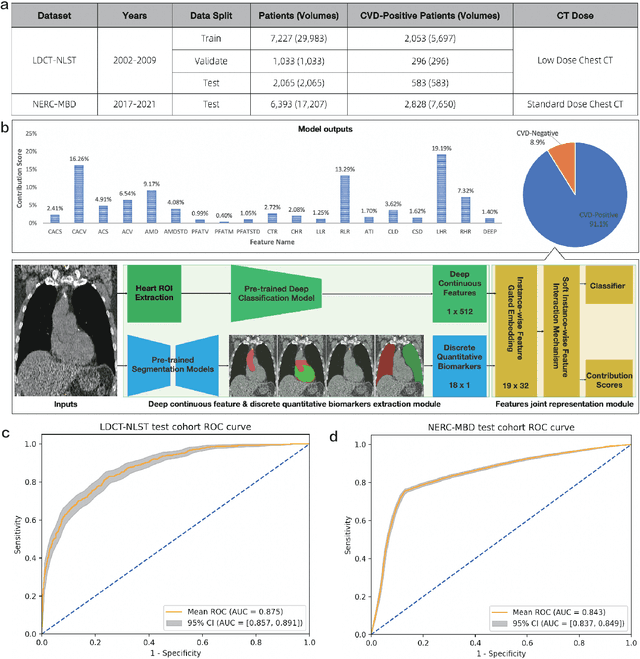
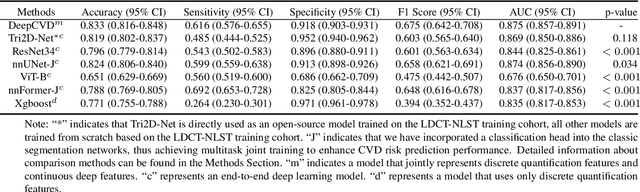
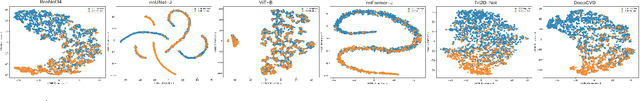
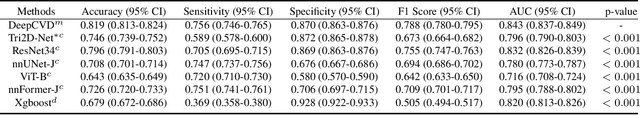
Abstract:Cardiovascular diseases (CVD) remain a leading health concern and contribute significantly to global mortality rates. While clinical advancements have led to a decline in CVD mortality, accurately identifying individuals who could benefit from preventive interventions remains an unsolved challenge in preventive cardiology. Current CVD risk prediction models, recommended by guidelines, are based on limited traditional risk factors or use CT imaging to acquire quantitative biomarkers, and still have limitations in predictive accuracy and applicability. On the other hand, end-to-end trained CVD risk prediction methods leveraging deep learning on CT images often fail to provide transparent and explainable decision grounds for assisting physicians. In this work, we proposed a novel joint representation that integrates discrete quantitative biomarkers and continuous deep features extracted from chest CT scans. Our approach initiated with a deep CVD risk classification model by capturing comprehensive continuous deep learning features while jointly obtaining currently clinical-established quantitative biomarkers via segmentation models. In the feature joint representation stage, we use an instance-wise feature-gated mechanism to align the continuous and discrete features, followed by a soft instance-wise feature interaction mechanism fostering independent and effective feature interaction for the final CVD risk prediction. Our method substantially improves CVD risk predictive performance and offers individual contribution analysis of each biomarker, which is important in assisting physicians' decision-making processes. We validated our method on a public chest low-dose CT dataset and a private external chest standard-dose CT patient cohort of 17,207 CT volumes from 6,393 unique subjects, and demonstrated superior predictive performance, achieving AUCs of 0.875 and 0.843, respectively.
2020 CATARACTS Semantic Segmentation Challenge
Oct 21, 2021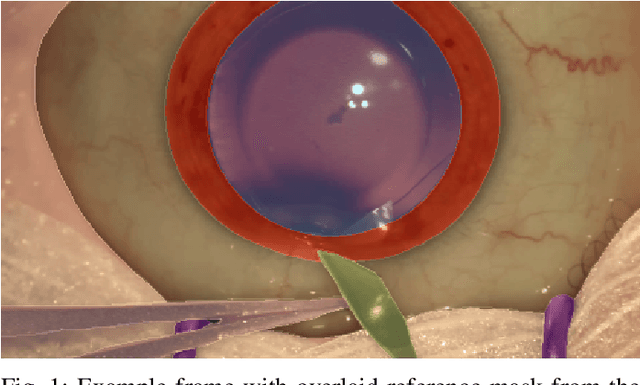
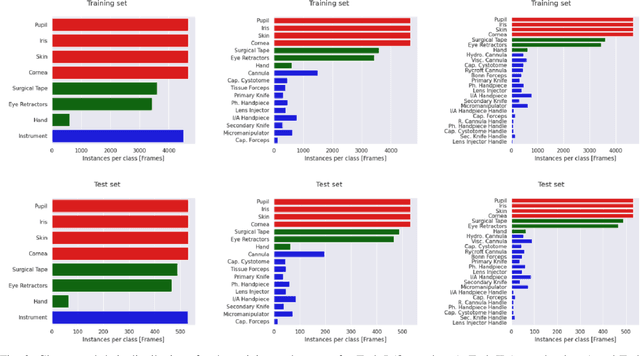
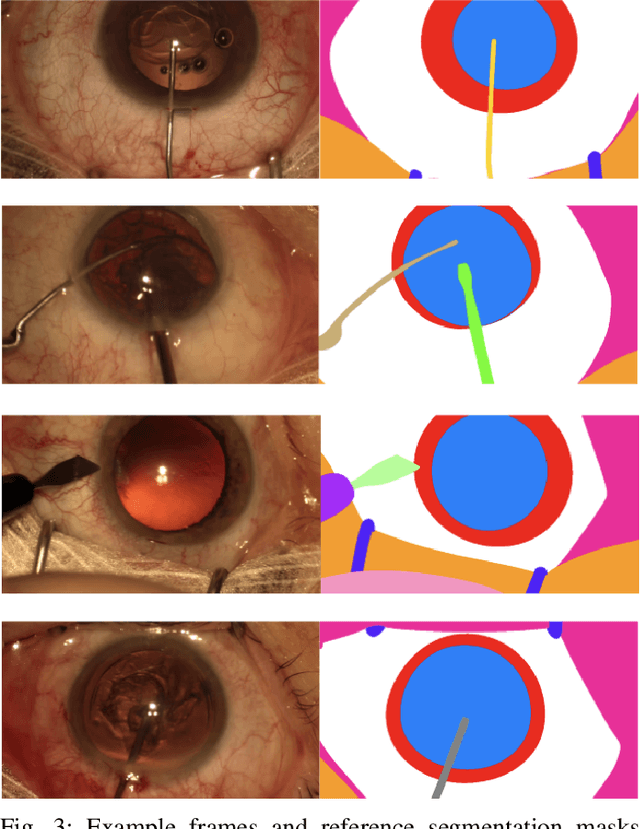
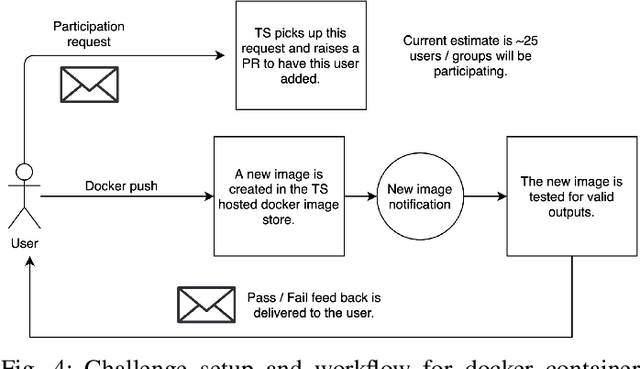
Abstract:Surgical scene segmentation is essential for anatomy and instrument localization which can be further used to assess tissue-instrument interactions during a surgical procedure. In 2017, the Challenge on Automatic Tool Annotation for cataRACT Surgery (CATARACTS) released 50 cataract surgery videos accompanied by instrument usage annotations. These annotations included frame-level instrument presence information. In 2020, we released pixel-wise semantic annotations for anatomy and instruments for 4670 images sampled from 25 videos of the CATARACTS training set. The 2020 CATARACTS Semantic Segmentation Challenge, which was a sub-challenge of the 2020 MICCAI Endoscopic Vision (EndoVis) Challenge, presented three sub-tasks to assess participating solutions on anatomical structure and instrument segmentation. Their performance was assessed on a hidden test set of 531 images from 10 videos of the CATARACTS test set.
Group Feature Learning and Domain Adversarial Neural Network for aMCI Diagnosis System Based on EEG
Apr 28, 2021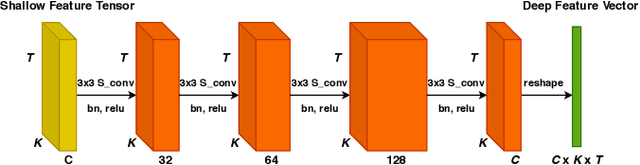
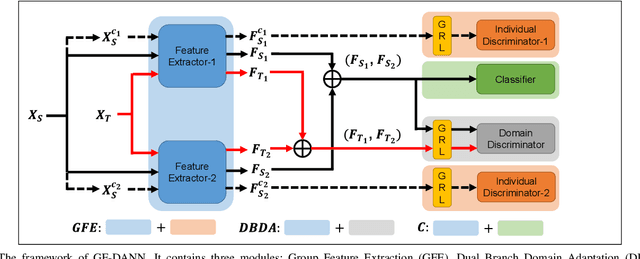
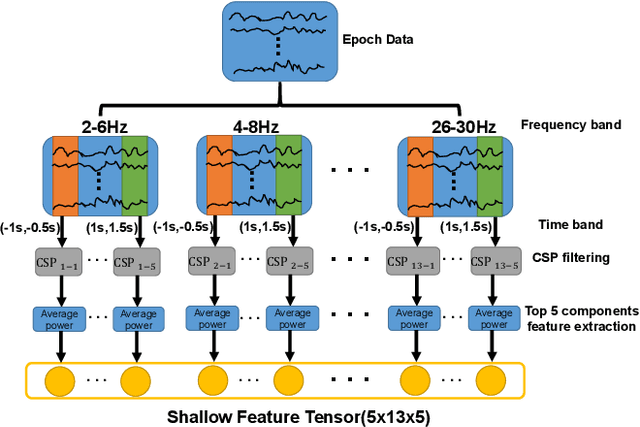
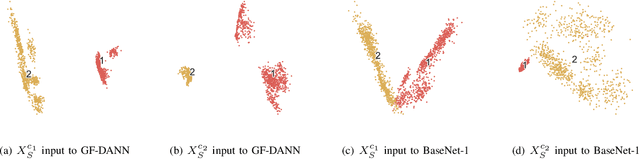
Abstract:Medical diagnostic robot systems have been paid more and more attention due to its objectivity and accuracy. The diagnosis of mild cognitive impairment (MCI) is considered an effective means to prevent Alzheimer's disease (AD). Doctors diagnose MCI based on various clinical examinations, which are expensive and the diagnosis results rely on the knowledge of doctors. Therefore, it is necessary to develop a robot diagnostic system to eliminate the influence of human factors and obtain a higher accuracy rate. In this paper, we propose a novel Group Feature Domain Adversarial Neural Network (GF-DANN) for amnestic MCI (aMCI) diagnosis, which involves two important modules. A Group Feature Extraction (GFE) module is proposed to reduce individual differences by learning group-level features through adversarial learning. A Dual Branch Domain Adaptation (DBDA) module is carefully designed to reduce the distribution difference between the source and target domain in a domain adaption way. On three types of data set, GF-DANN achieves the best accuracy compared with classic machine learning and deep learning methods. On the DMS data set, GF-DANN has obtained an accuracy rate of 89.47%, and the sensitivity and specificity are 90% and 89%. In addition, by comparing three EEG data collection paradigms, our results demonstrate that the DMS paradigm has the potential to build an aMCI diagnose robot system.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge