Chaoping Zhang
Deep MRI Reconstruction with Radial Subsampling
Aug 20, 2021



Abstract:In spite of its extensive adaptation in almost every medical diagnostic and examinatorial application, Magnetic Resonance Imaging (MRI) is still a slow imaging modality which limits its use for dynamic imaging. In recent years, Parallel Imaging (PI) and Compressed Sensing (CS) have been utilised to accelerate the MRI acquisition. In clinical settings, subsampling the k-space measurements during scanning time using Cartesian trajectories, such as rectilinear sampling, is currently the most conventional CS approach applied which, however, is prone to producing aliased reconstructions. With the advent of the involvement of Deep Learning (DL) in accelerating the MRI, reconstructing faithful images from subsampled data became increasingly promising. Retrospectively applying a subsampling mask onto the k-space data is a way of simulating the accelerated acquisition of k-space data in real clinical setting. In this paper we compare and provide a review for the effect of applying either rectilinear or radial retrospective subsampling on the quality of the reconstructions outputted by trained deep neural networks. With the same choice of hyper-parameters, we train and evaluate two distinct Recurrent Inference Machines (RIMs), one for each type of subsampling. The qualitative and quantitative results of our experiments indicate that the model trained on data with radial subsampling attains higher performance and learns to estimate reconstructions with higher fidelity paving the way for other DL approaches to involve radial subsampling.
State-of-the-Art Machine Learning MRI Reconstruction in 2020: Results of the Second fastMRI Challenge
Dec 28, 2020
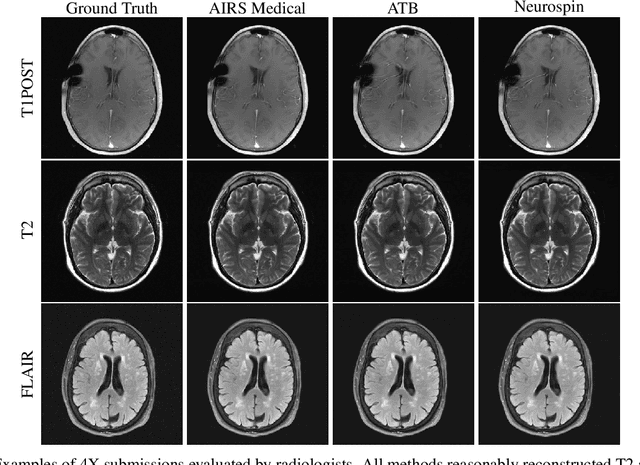
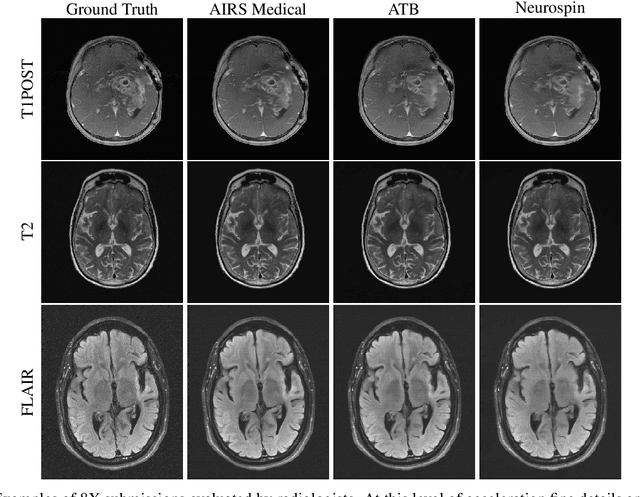
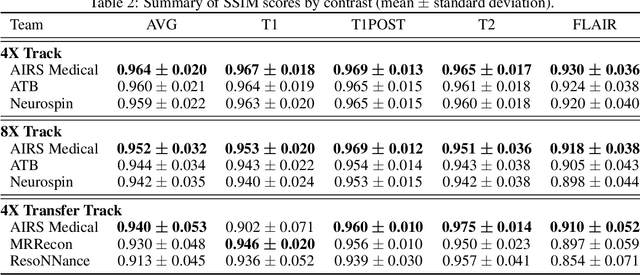
Abstract:Accelerating MRI scans is one of the principal outstanding problems in the MRI research community. Towards this goal, we hosted the second fastMRI competition targeted towards reconstructing MR images with subsampled k-space data. We provided participants with data from 7,299 clinical brain scans (de-identified via a HIPAA-compliant procedure by NYU Langone Health), holding back the fully-sampled data from 894 of these scans for challenge evaluation purposes. In contrast to the 2019 challenge, we focused our radiologist evaluations on pathological assessment in brain images. We also debuted a new Transfer track that required participants to submit models evaluated on MRI scanners from outside the training set. We received 19 submissions from eight different groups. Results showed one team scoring best in both SSIM scores and qualitative radiologist evaluations. We also performed analysis on alternative metrics to mitigate the effects of background noise and collected feedback from the participants to inform future challenges. Lastly, we identify common failure modes across the submissions, highlighting areas of need for future research in the MRI reconstruction community.
APIR-Net: Autocalibrated Parallel Imaging Reconstruction using a Neural Network
Sep 19, 2019



Abstract:Deep learning has been successfully demonstrated in MRI reconstruction of accelerated acquisitions. However, its dependence on representative training data limits the application across different contrasts, anatomies, or image sizes. To address this limitation, we propose an unsupervised, auto-calibrated k-space completion method, based on a uniquely designed neural network that reconstructs the full k-space from an undersampled k-space, exploiting the redundancy among the multiple channels in the receive coil in a parallel imaging acquisition. To achieve this, contrary to common convolutional network approaches, the proposed network has a decreasing number of feature maps of constant size. In contrast to conventional parallel imaging methods such as GRAPPA that estimate the prediction kernel from the fully sampled autocalibration signals in a linear way, our method is able to learn nonlinear relations between sampled and unsampled positions in k-space. The proposed method was compared to the start-of-the-art ESPIRiT and RAKI methods in terms of noise amplification and visual image quality in both phantom and in-vivo experiments. The experiments indicate that APIR-Net provides a promising alternative to the conventional parallel imaging methods, and results in improved image quality especially for low SNR acquisitions.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge