Bingxue Wang
Online 4D Ultrasound-Guided Robotic Tracking Enables 3D Ultrasound Localisation Microscopy with Large Tissue Displacements
Sep 17, 2024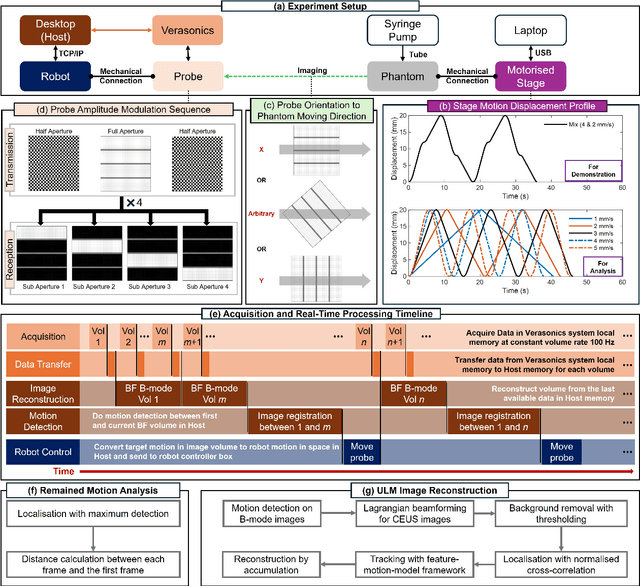
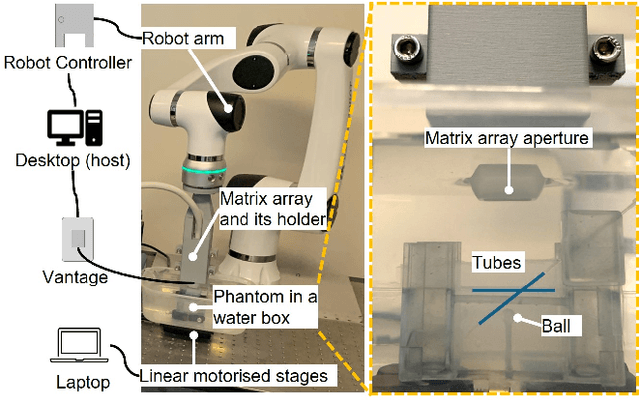
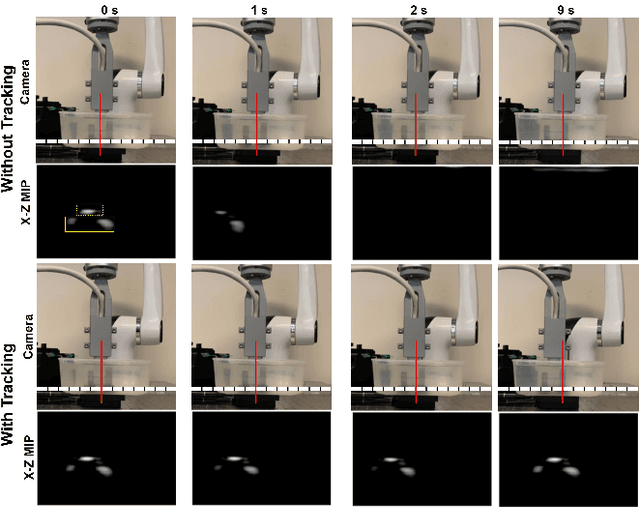
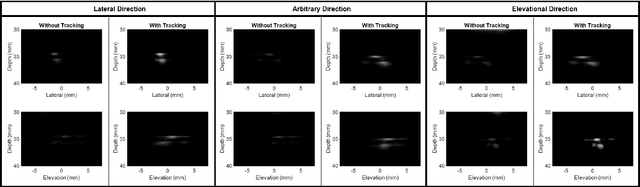
Abstract:Super-Resolution Ultrasound (SRUS) imaging through localising and tracking microbubbles, also known as Ultrasound Localisation Microscopy (ULM), has demonstrated significant potential for reconstructing microvasculature and flows with sub-diffraction resolution in clinical diagnostics. However, imaging organs with large tissue movements, such as those caused by respiration, presents substantial challenges. Existing methods often require breath holding to maintain accumulation accuracy, which limits data acquisition time and ULM image saturation. To improve image quality in the presence of large tissue movements, this study introduces an approach integrating high-frame-rate ultrasound with online precise robotic probe control. Tested on a microvasculature phantom with translation motions up to 20 mm, twice the aperture size of the matrix array used, our method achieved real-time tracking of the moving phantom and imaging volume rate at 85 Hz, keeping majority of the target volume in the imaging field of view. ULM images of the moving cross channels in the phantom were successfully reconstructed in post-processing, demonstrating the feasibility of super-resolution imaging under large tissue motions. This represents a significant step towards ULM imaging of organs with large motion.
Enhancing super-resolution ultrasound localisation through multi-frame deconvolution exploiting spatiotemporal coherence
Jul 08, 2024Abstract:Super-resolution ultrasound imaging through microbubble (MB) localisation and tracking, also known as ultrasound localisation microscopy, allows non-invasive sub-diffraction resolution imaging of microvasculature in animals and humans. The number of MBs localised from the acquired contrast-enhanced ultrasound (CEUS) images and the localisation precision directly influence the quality of the resulting super-resolution microvasculature images. However, non-negligible noise present in the CEUS images can make localising MBs challenging. To enhance the MB localisation performance, we propose a Multi-Frame Deconvolution (MF-Decon) framework that can exploit the spatiotemporal coherence inherent in the CEUS data, with new spatial and temporal regularisers designed based on total variation (TV) and regularisation by denoising (RED). Based on the MF-Decon framework, we introduce two novel methods: MF-Decon with spatial and temporal TVs (MF-Decon+3DTV) and MF-Decon with spatial RED and temporal TV (MF-Decon+RED+TV). Results from in silico simulations indicate that our methods outperform two widely used methods using deconvolution or normalised cross-correlation across all evaluation metrics, including precision, recall, $F_1$ score, mean and standard localisation errors. In particular, our methods improve MB localisation precision by up to 39% and recall by up to 12%. Super-resolution microvasculature maps generated with our methods on a publicly available in vivo rat brain dataset show less noise, better contrast, higher resolution and more vessel structures.
A Weakly Supervised Segmentation Network Embedding Cross-scale Attention Guidance and Noise-sensitive Constraint for Detecting Tertiary Lymphoid Structures of Pancreatic Tumors
Jul 27, 2023


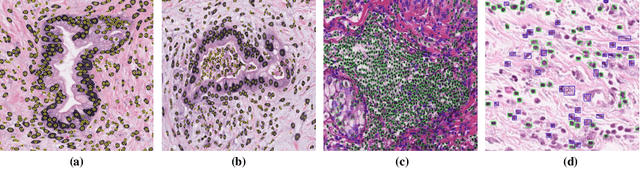
Abstract:The presence of tertiary lymphoid structures (TLSs) on pancreatic pathological images is an important prognostic indicator of pancreatic tumors. Therefore, TLSs detection on pancreatic pathological images plays a crucial role in diagnosis and treatment for patients with pancreatic tumors. However, fully supervised detection algorithms based on deep learning usually require a large number of manual annotations, which is time-consuming and labor-intensive. In this paper, we aim to detect the TLSs in a manner of few-shot learning by proposing a weakly supervised segmentation network. We firstly obtain the lymphocyte density maps by combining a pretrained model for nuclei segmentation and a domain adversarial network for lymphocyte nuclei recognition. Then, we establish a cross-scale attention guidance mechanism by jointly learning the coarse-scale features from the original histopathology images and fine-scale features from our designed lymphocyte density attention. A noise-sensitive constraint is introduced by an embedding signed distance function loss in the training procedure to reduce tiny prediction errors. Experimental results on two collected datasets demonstrate that our proposed method significantly outperforms the state-of-the-art segmentation-based algorithms in terms of TLSs detection accuracy. Additionally, we apply our method to study the congruent relationship between the density of TLSs and peripancreatic vascular invasion and obtain some clinically statistical results.
BUbble Flow Field: a Simulation Framework for Evaluating Ultrasound Localization Microscopy Algorithms
Nov 01, 2022



Abstract:Ultrasound contrast enhanced imaging has seen widespread uptake in research and clinical diagnostic imaging. This includes applications such as vector flow imaging, functional ultrasound and super-resolution Ultrasound Localization Microscopy (ULM). All of these require testing and validation during development of new algorithms with ground truth data. In this work we present a comprehensive simulation platform BUbble Flow Field (BUFF) that generates contrast enhanced ultrasound images in vascular tree geometries with realistic flow characteristics and validation algorithms for ULM. BUFF allows complex micro-vascular network generation of random and user-defined vascular networks. Blood flow is simulated with a fast Computational Fluid Dynamics (CFD) solver and allows arbitrary input and output positions and custom pressures. The acoustic field simulation is combined with non-linear Microbubble (MB) dynamics and simulates a range of point spread functions based on user-defined MB characteristics. The validation combines both binary and quantitative metrics. BFF's capacity to generate and validate user-defined networks is demonstrated through its implementation in the Ultrasound Localisation and TRacking Algorithms for Super Resolution (ULTRA-SR) Challenge at the International Ultrasonics Symposium (IUS) 2022 of the Institute of Electrical and Electronics Engineers (IEEE). The ability to produce ULM images, and the availability of a ground truth in localisation and tracking enables objective and quantitative evaluation of the large number of localisation and tracking algorithms developed in the field. BUFF can also benefit deep learning based methods by automatically generating datasets for training. BUFF is a fully comprehensive simulation platform for testing and validation of novel ULM techniques and is open source.
3D Super-Resolution Ultrasound with Adaptive Weight-Based Beamforming
Aug 25, 2022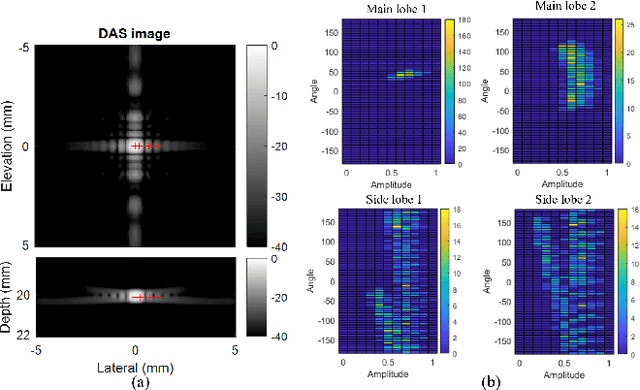
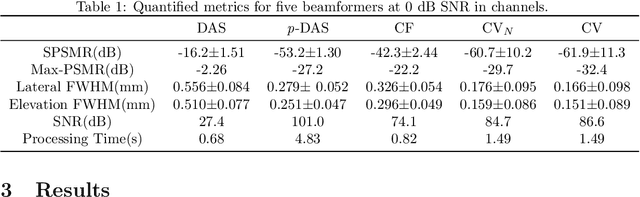
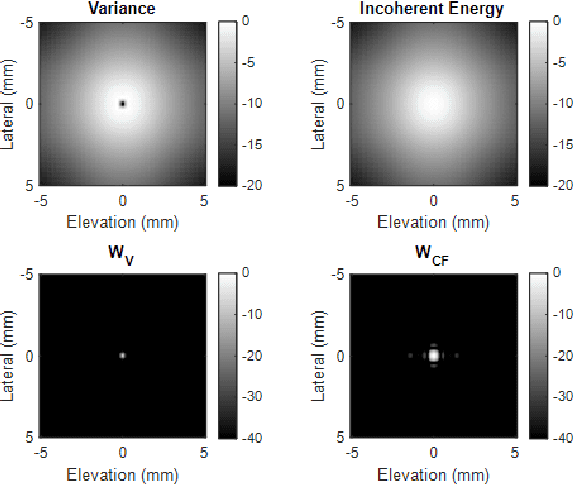
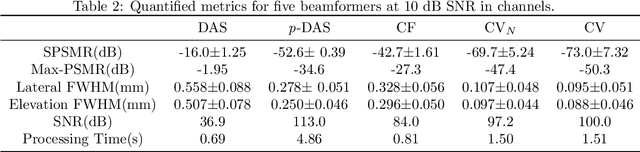
Abstract:Super-resolution ultrasound (SRUS) imaging through localising and tracking sparse microbubbles has been shown to reveal microvascular structure and flow beyond the wave diffraction limit. Most SRUS studies use standard delay and sum (DAS) beamforming, where large main lobe and significant side lobes make separation and localisation of densely distributed bubbles challenging, particularly in 3D due to the typically small aperture of matrix array probes. This study aims to improve 3D SRUS by implementing a low-cost 3D coherence beamformer based on channel signal variance, as well as two other adaptive weight-based coherence beamformers: nonlinear beamforming with p-th root compression and coherence factor. The 3D coherence beamformers, together with DAS, are compared in computer simulation, on a microflow phantom, and in vivo. Simulation results demonstrate that the adaptive weight-based beamformers can significantly narrow the main lobe and suppress the side lobes for modest computational cost. Significantly improved 3D SR images of microflow phantom and a rabbit kidney are obtained through the adaptive weight-based beamformers. The proposed variance-based beamformer performs best in simulations and experiments.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge