Qingyuan Tan
Online 4D Ultrasound-Guided Robotic Tracking Enables 3D Ultrasound Localisation Microscopy with Large Tissue Displacements
Sep 17, 2024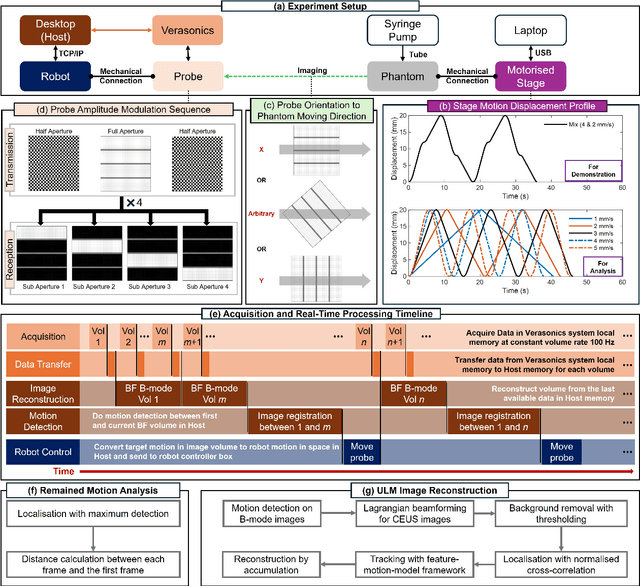
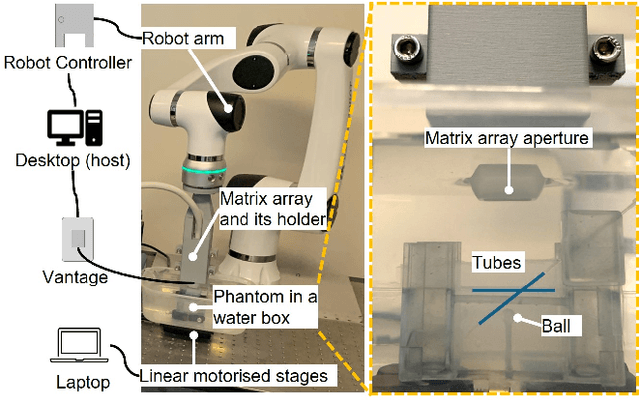
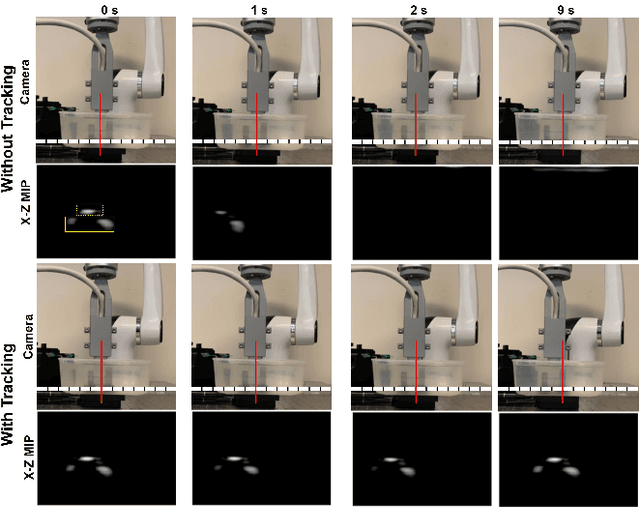
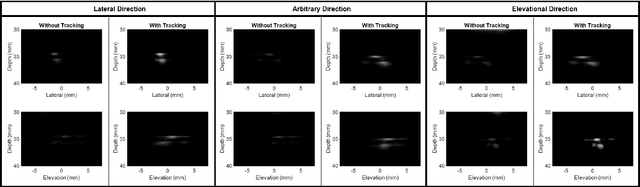
Abstract:Super-Resolution Ultrasound (SRUS) imaging through localising and tracking microbubbles, also known as Ultrasound Localisation Microscopy (ULM), has demonstrated significant potential for reconstructing microvasculature and flows with sub-diffraction resolution in clinical diagnostics. However, imaging organs with large tissue movements, such as those caused by respiration, presents substantial challenges. Existing methods often require breath holding to maintain accumulation accuracy, which limits data acquisition time and ULM image saturation. To improve image quality in the presence of large tissue movements, this study introduces an approach integrating high-frame-rate ultrasound with online precise robotic probe control. Tested on a microvasculature phantom with translation motions up to 20 mm, twice the aperture size of the matrix array used, our method achieved real-time tracking of the moving phantom and imaging volume rate at 85 Hz, keeping majority of the target volume in the imaging field of view. ULM images of the moving cross channels in the phantom were successfully reconstructed in post-processing, demonstrating the feasibility of super-resolution imaging under large tissue motions. This represents a significant step towards ULM imaging of organs with large motion.
Ultrafast 3-D Super Resolution Ultrasound using Row-Column Array specific Coherence-based Beamforming and Rolling Acoustic Sub-aperture Processing: In Vitro, In Vivo and Clinical Study
Nov 15, 2023



Abstract:The row-column addressed array is an emerging probe for ultrafast 3-D ultrasound imaging. It achieves this with far fewer independent electronic channels and a wider field of view than traditional 2-D matrix arrays, of the same channel count, making it a good candidate for clinical translation. However, the image quality of row-column arrays is generally poor, particularly when investigating tissue. Ultrasound localisation microscopy allows for the production of super-resolution images even when the initial image resolution is not high. Unfortunately, the row-column probe can suffer from imaging artefacts that can degrade the quality of super-resolution images as `secondary' lobes from bright microbubbles can be mistaken as microbubble events, particularly when operated using plane wave imaging. These false events move through the image in a physiologically realistic way so can be challenging to remove via tracking, leading to the production of 'false vessels'. Here, a new type of rolling window image reconstruction procedure was developed, which integrated a row-column array-specific coherence-based beamforming technique with acoustic sub-aperture processing for the purposes of reducing `secondary' lobe artefacts, noise and increasing the effective frame rate. Using an {\it{in vitro}} cross tube, it was found that the procedure reduced the percentage of `false' locations from $\sim$26\% to $\sim$15\% compared to traditional orthogonal plane wave compounding. Additionally, it was found that the noise could be reduced by $\sim$7 dB and that the effective frame rate could be increased to over 4000 fps. Subsequently, {\it{in vivo}} ultrasound localisation microscopy was used to produce images non-invasively of a rabbit kidney and a human thyroid.
Transthoracic super-resolution ultrasound localisation microscopy of myocardial vasculature in patients
Mar 28, 2023Abstract:Micro-vascular flow in the myocardium is of significant importance clinically but remains poorly understood. Up to 25% of patients with symptoms of coronary heart diseases have no obstructive coronary arteries and have suspected microvascular diseases. However, such microvasculature is difficult to image in vivo with existing modalities due to the lack of resolution and sensitivity. Here, we demonstrate the feasibility of transthoracic super-resolution ultrasound localisation microscopy (SRUS/ULM) of myocardial microvasculature and hemodynamics in a large animal model and in patients, using a cardiac phased array probe with a customised data acquisition and processing pipeline. A multi-level motion correction strategy was proposed. A tracking framework incorporating multiple features and automatic parameter initialisations was developed to reconstruct microcirculation. In two patients with impaired myocardial function, we have generated SRUS images of myocardial vascular structure and flow with a resolution that is beyond the wave-diffraction limit (half a wavelength), using data acquired within a breath hold. Myocardial SRUS/ULM has potential to improve the understanding of myocardial microcirculation and the management of patients with cardiac microvascular diseases.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge