Konstantinos Ntagiantas
Department of Bioengineering, Imperial College London
Transthoracic super-resolution ultrasound localisation microscopy of myocardial vasculature in patients
Mar 28, 2023Abstract:Micro-vascular flow in the myocardium is of significant importance clinically but remains poorly understood. Up to 25% of patients with symptoms of coronary heart diseases have no obstructive coronary arteries and have suspected microvascular diseases. However, such microvasculature is difficult to image in vivo with existing modalities due to the lack of resolution and sensitivity. Here, we demonstrate the feasibility of transthoracic super-resolution ultrasound localisation microscopy (SRUS/ULM) of myocardial microvasculature and hemodynamics in a large animal model and in patients, using a cardiac phased array probe with a customised data acquisition and processing pipeline. A multi-level motion correction strategy was proposed. A tracking framework incorporating multiple features and automatic parameter initialisations was developed to reconstruct microcirculation. In two patients with impaired myocardial function, we have generated SRUS images of myocardial vascular structure and flow with a resolution that is beyond the wave-diffraction limit (half a wavelength), using data acquired within a breath hold. Myocardial SRUS/ULM has potential to improve the understanding of myocardial microcirculation and the management of patients with cardiac microvascular diseases.
Estimating Cardiac Tissue Conductivity from Electrograms with Fully Convolutional Networks
Dec 06, 2022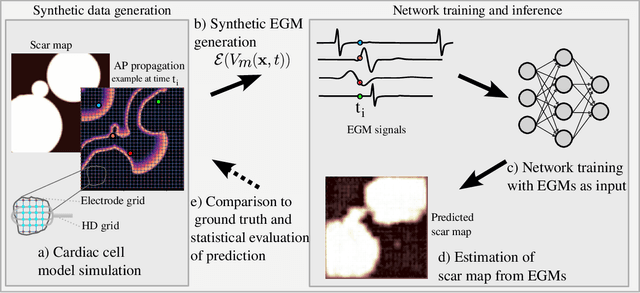
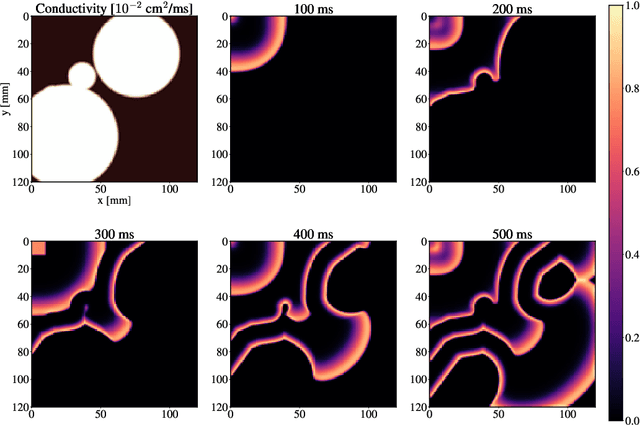
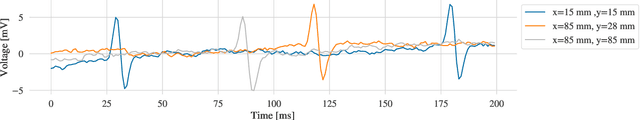
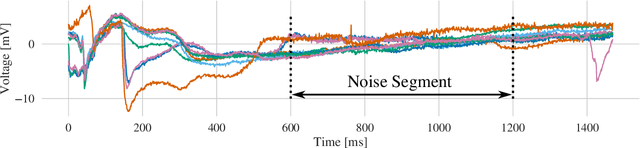
Abstract:Atrial Fibrillation (AF) is characterized by disorganised electrical activity in the atria and is known to be sustained by the presence of regions of fibrosis (scars) or functional cellular remodeling, both of which may lead to areas of slow conduction. Estimating the effective conductivity of the myocardium and identifying regions of abnormal propagation is therefore crucial for the effective treatment of AF. We hypothesise that the spatial distribution of tissue conductivity can be directly inferred from an array of concurrently acquired contact electrograms (EGMs). We generate a dataset of simulated cardiac AP propagation using randomised scar distributions and a phenomenological cardiac model and calculate contact electrograms at various positions on the field. A deep neural network, based on a modified U-net architecture, is trained to estimate the location of the scar and quantify conductivity of the tissue with a Jaccard index of $91$%. We adapt a wavelet-based surrogate testing analysis to confirm that the inferred conductivity distribution is an accurate representation of the ground truth input to the model. We find that the root mean square error (RMSE) between the ground truth and our predictions is significantly smaller ($p_{val}=0.007$) than the RMSE between the ground truth and surrogate samples.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge