Peter D. Weinberg
Enhanced Acoustic Beamforming with Sub-Aperture Angular Multiply and Sum -- in vivo and in Human Demonstration
Jan 10, 2025



Abstract:Power Doppler ultrasound is in widespread clinical use for non-invasive vascular imaging but the most common current method - Delay and Sum (DAS) beamforming - suffers from limited resolution and high side-lobes. Here we propose the Sub-Aperture Angular Multiply and Sum (SAMAS) algorithm; it combines the advantages of two recent non-linear beamformers, Frame Multiply and Sum (FMAS) which uses signal temporal coherence and the acoustic sub-aperture (ASAP) algorithm, which uses signal spatial coherence, respectively. Following in vitro experiments to optimise the algorithm, particularly the use of phase information and sub-aperture pairing, it was evaluated in vivo, first in a rabbit kidney and then in human lymph node, using ultrafast ultrasound images obtained with intravenous contrast agents. The SAMAS algorithm improved the CNR and SNR across all tests, on average raising the CNR by 11 dB and the SNR by 18 dB over DAS in vivo. This work demonstrates a promising vascular imaging method that could have widespread clinical utility.
Ultrafast 3-D Super Resolution Ultrasound using Row-Column Array specific Coherence-based Beamforming and Rolling Acoustic Sub-aperture Processing: In Vitro, In Vivo and Clinical Study
Nov 15, 2023



Abstract:The row-column addressed array is an emerging probe for ultrafast 3-D ultrasound imaging. It achieves this with far fewer independent electronic channels and a wider field of view than traditional 2-D matrix arrays, of the same channel count, making it a good candidate for clinical translation. However, the image quality of row-column arrays is generally poor, particularly when investigating tissue. Ultrasound localisation microscopy allows for the production of super-resolution images even when the initial image resolution is not high. Unfortunately, the row-column probe can suffer from imaging artefacts that can degrade the quality of super-resolution images as `secondary' lobes from bright microbubbles can be mistaken as microbubble events, particularly when operated using plane wave imaging. These false events move through the image in a physiologically realistic way so can be challenging to remove via tracking, leading to the production of 'false vessels'. Here, a new type of rolling window image reconstruction procedure was developed, which integrated a row-column array-specific coherence-based beamforming technique with acoustic sub-aperture processing for the purposes of reducing `secondary' lobe artefacts, noise and increasing the effective frame rate. Using an {\it{in vitro}} cross tube, it was found that the procedure reduced the percentage of `false' locations from $\sim$26\% to $\sim$15\% compared to traditional orthogonal plane wave compounding. Additionally, it was found that the noise could be reduced by $\sim$7 dB and that the effective frame rate could be increased to over 4000 fps. Subsequently, {\it{in vivo}} ultrasound localisation microscopy was used to produce images non-invasively of a rabbit kidney and a human thyroid.
On the Use of Singular Value Decomposition as a Clutter Filter for Ultrasound Flow Imaging
Apr 25, 2023



Abstract:Filtering based on Singular Value Decomposition (SVD) provides substantial separation of clutter, flow and noise in high frame rate ultrasound flow imaging. The use of SVD as a clutter filter has greatly improved techniques such as vector flow imaging, functional ultrasound and super-resolution ultrasound localization microscopy. The removal of clutter and noise relies on the assumption that tissue, flow and noise are each represented by different subsets of singular values, so that their signals are uncorrelated and lay on orthogonal sub-spaces. This assumption fails in the presence of tissue motion, for near-wall or microvascular flow, and can be influenced by an incorrect choice of singular value thresholds. Consequently, separation of flow, clutter and noise is imperfect, which can lead to image artefacts not present in the original data. Temporal and spatial fluctuation in intensity are the commonest artefacts, which vary in appearance and strengths. Ghosting and splitting artefacts are observed in the microvasculature where the flow signal is sparsely distributed. Singular value threshold selection, tissue motion, frame rate, flow signal amplitude and acquisition length affect the prevalence of these artefacts. Understanding what causes artefacts due to SVD clutter and noise removal is necessary for their interpretation.
3D Super-Resolution Ultrasound with Adaptive Weight-Based Beamforming
Aug 25, 2022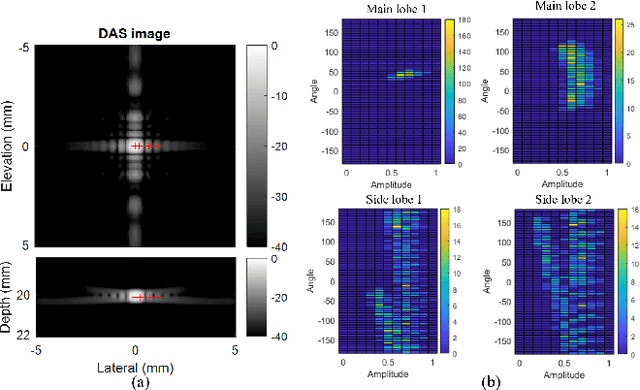
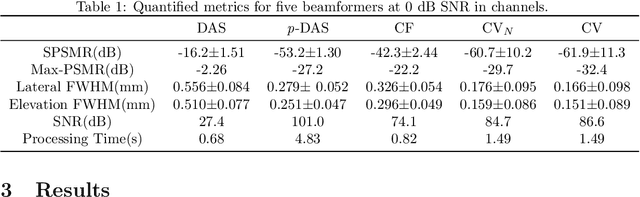
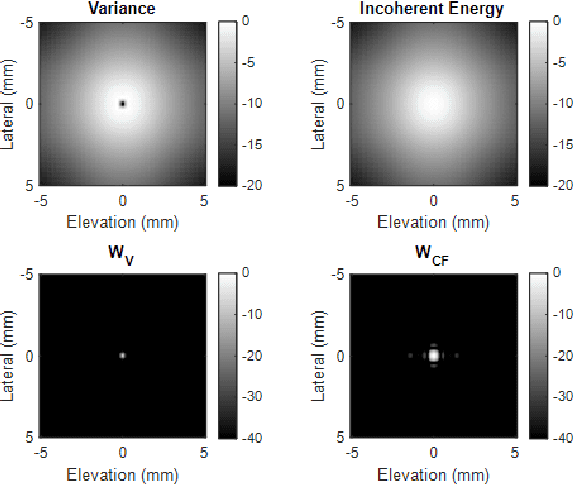
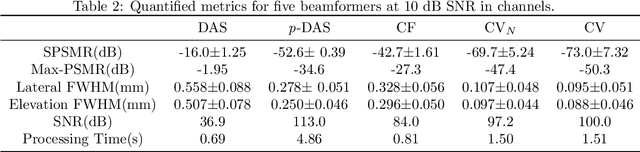
Abstract:Super-resolution ultrasound (SRUS) imaging through localising and tracking sparse microbubbles has been shown to reveal microvascular structure and flow beyond the wave diffraction limit. Most SRUS studies use standard delay and sum (DAS) beamforming, where large main lobe and significant side lobes make separation and localisation of densely distributed bubbles challenging, particularly in 3D due to the typically small aperture of matrix array probes. This study aims to improve 3D SRUS by implementing a low-cost 3D coherence beamformer based on channel signal variance, as well as two other adaptive weight-based coherence beamformers: nonlinear beamforming with p-th root compression and coherence factor. The 3D coherence beamformers, together with DAS, are compared in computer simulation, on a microflow phantom, and in vivo. Simulation results demonstrate that the adaptive weight-based beamformers can significantly narrow the main lobe and suppress the side lobes for modest computational cost. Significantly improved 3D SR images of microflow phantom and a rabbit kidney are obtained through the adaptive weight-based beamformers. The proposed variance-based beamformer performs best in simulations and experiments.
Fast and selective super-resolution ultrasound in vivo with sono-switchable nanodroplets
Mar 08, 2022



Abstract:Perfusion by the microcirculation is key to the development, maintenance and pathology of tissue. Its measurement with high spatiotemporal resolution is consequently valuable but remains a challenge in deep tissue. Ultrasound Localization Microscopy (ULM) provides very high spatiotemporal resolution but the use of microbubbles requires low contrast agent concentrations, a long acquisition time, and gives little control over the spatial and temporal distribution of the bubbles. The present study is the first to demonstrate Acoustic Wave Sparsely-Activated Localization Microscopy (AWSALM) and fast-AWSALM for in vivo super-resolution ultrasound imaging, offering contrast on demand and vascular selectivity. Three different formulations of sono-switchable contrast agents were tested. We demonstrate their use with ultrasound mechanical indices well within recommended safety limits to enable fast on-demand sparse switching at very high agent concentrations. We produce super-localization maps of the rabbit renal vasculature with acquisition times between 5.5 s and 0.25 s, and an 4-fold improvement in spatial resolution. We present the unique selectivity of AWSALM in visualizing specific vascular branches and downstream microvasculature, and we show super-localized kidney structures in systole and diastole with fast-AWSALM. In conclusion we demonstrate the feasibility of fast and selective measurement of microvascular dynamics in vivo with subwavelength resolution using ultrasound and sono-switchable nanodroplets.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge