Beomseok Sohn
Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
Large Language Models are Clinical Reasoners: Reasoning-Aware Diagnosis Framework with Prompt-Generated Rationales
Dec 12, 2023Abstract:Machine reasoning has made great progress in recent years owing to large language models (LLMs). In the clinical domain, however, most NLP-driven projects mainly focus on clinical classification or reading comprehension, and under-explore clinical reasoning for disease diagnosis due to the expensive rationale annotation with clinicians. In this work, we present a ``reasoning-aware'' diagnosis framework that rationalizes the diagnostic process via prompt-based learning in a time- and labor-efficient manner, and learns to reason over the prompt-generated rationales. Specifically, we address the clinical reasoning for disease diagnosis, where the LLM generates diagnostic rationales providing its insight on presented patient data and the reasoning path towards the diagnosis, namely Clinical Chain-of-Thought (Clinical CoT). We empirically demonstrate LLMs/LMs' ability of clinical reasoning via extensive experiments and analyses on both rationale generation and disease diagnosis in various settings. We further propose a novel set of criteria for evaluating machine-generated rationales' potential for real-world clinical settings, facilitating and benefiting future research in this area.
A human brain atlas of chi-separation for normative iron and myelin distributions
Nov 08, 2023Abstract:Iron and myelin are primary susceptibility sources in the human brain. These substances are essential for healthy brain, and their abnormalities are often related to various neurological disorders. Recently, an advanced susceptibility mapping technique, which is referred to as chi-separation, has been proposed successfully disentangling paramagnetic iron from diamagnetic myelin, opening a new potential for generating iron map and myelin map in the brain. Utilizing this technique, this study constructs a normative chi-separation atlas from 106 healthy human brains. The resulting atlas provides detailed anatomical structures associated with the distributions of iron and myelin, clearly delineating subcortical nuclei and white matter fiber bundles. Additionally, susceptibility values in a number of regions of interest are reported along with age-dependent changes. This atlas may have direct applications such as localization of subcortical structures for deep brain stimulation or high-intensity focused ultrasound and also serve as a valuable resource for future research.
Evidence-empowered Transfer Learning for Alzheimer's Disease
Mar 03, 2023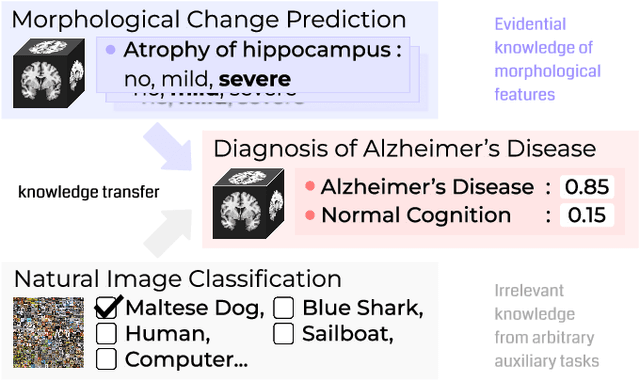
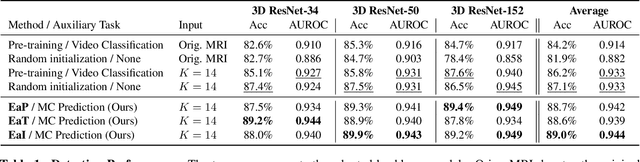
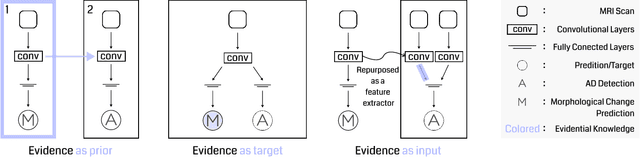
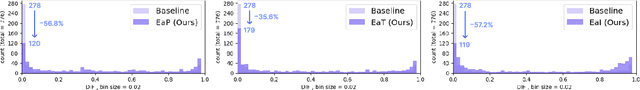
Abstract:Transfer learning has been widely utilized to mitigate the data scarcity problem in the field of Alzheimer's disease (AD). Conventional transfer learning relies on re-using models trained on AD-irrelevant tasks such as natural image classification. However, it often leads to negative transfer due to the discrepancy between the non-medical source and target medical domains. To address this, we present evidence-empowered transfer learning for AD diagnosis. Unlike conventional approaches, we leverage an AD-relevant auxiliary task, namely morphological change prediction, without requiring additional MRI data. In this auxiliary task, the diagnosis model learns the evidential and transferable knowledge from morphological features in MRI scans. Experimental results demonstrate that our framework is not only effective in improving detection performance regardless of model capacity, but also more data-efficient and faithful.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge