Anthony E. Sisk
Image Registration of In Vivo Micro-Ultrasound and Ex Vivo Pseudo-Whole Mount Histopathology Images of the Prostate: A Proof-of-Concept Study
May 31, 2023



Abstract:Early diagnosis of prostate cancer significantly improves a patient's 5-year survival rate. Biopsy of small prostate cancers is improved with image-guided biopsy. MRI-ultrasound fusion-guided biopsy is sensitive to smaller tumors but is underutilized due to the high cost of MRI and fusion equipment. Micro-ultrasound (micro-US), a novel high-resolution ultrasound technology, provides a cost-effective alternative to MRI while delivering comparable diagnostic accuracy. However, the interpretation of micro-US is challenging due to subtle gray scale changes indicating cancer vs normal tissue. This challenge can be addressed by training urologists with a large dataset of micro-US images containing the ground truth cancer outlines. Such a dataset can be mapped from surgical specimens (histopathology) onto micro-US images via image registration. In this paper, we present a semi-automated pipeline for registering in vivo micro-US images with ex vivo whole-mount histopathology images. Our pipeline begins with the reconstruction of pseudo-whole-mount histopathology images and a 3D micro-US volume. Each pseudo-whole-mount histopathology image is then registered with the corresponding axial micro-US slice using a two-stage approach that estimates an affine transformation followed by a deformable transformation. We evaluated our registration pipeline using micro-US and histopathology images from 18 patients who underwent radical prostatectomy. The results showed a Dice coefficient of 0.94 and a landmark error of 2.7 mm, indicating the accuracy of our registration pipeline. This proof-of-concept study demonstrates the feasibility of accurately aligning micro-US and histopathology images. To promote transparency and collaboration in research, we will make our code and dataset publicly available.
Deep learning-based transformation of the H&E stain into special stains improves kidney disease diagnosis
Aug 20, 2020
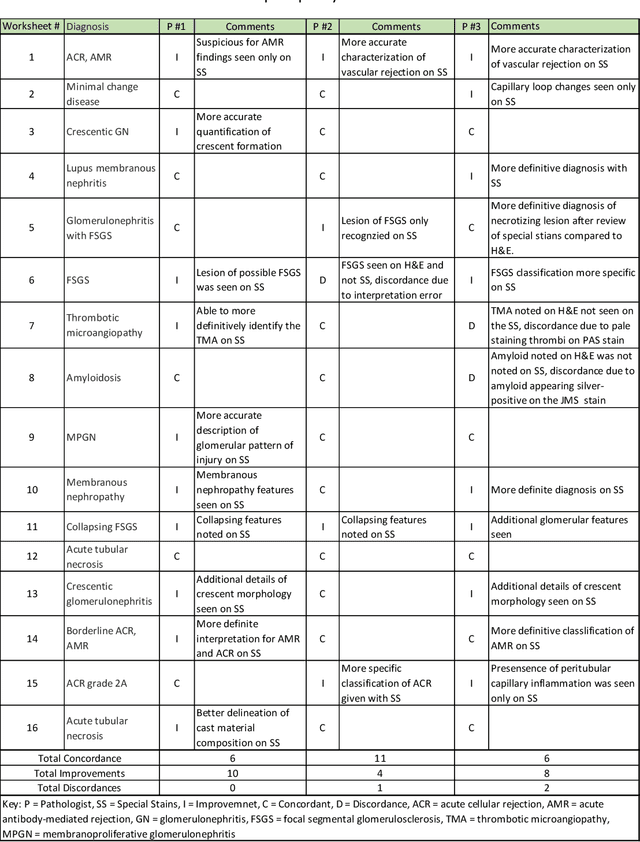


Abstract:Pathology is practiced by visual inspection of histochemically stained slides. Most commonly, the hematoxylin and eosin (H&E) stain is used in the diagnostic workflow and it is the gold standard for cancer diagnosis. However, in many cases, especially for non-neoplastic diseases, additional "special stains" are used to provide different levels of contrast and color to tissue components and allow pathologists to get a clearer diagnostic picture. In this study, we demonstrate the utility of supervised learning-based computational stain transformation from H&E to different special stains (Masson's Trichrome, periodic acid-Schiff and Jones silver stain) using tissue sections from kidney needle core biopsies. Based on evaluation by three renal pathologists, followed by adjudication by a fourth renal pathologist, we show that the generation of virtual special stains from existing H&E images improves the diagnosis in several non-neoplastic kidney diseases, sampled from 16 unique subjects. Adjudication of N=48 diagnoses from the three pathologists revealed that the virtually generated special stains yielded 22 improvements (45.8%), 23 concordances (47.9%) and 3 discordances (6.3%), when compared against the use of H&E stained tissue only. As the virtual transformation of H&E images into special stains can be achieved in less than 1 min per patient core specimen slide, this stain-to-stain transformation framework can improve the quality of the preliminary diagnosis when additional special stains are needed, along with significant savings in time and cost, reducing the burden on healthcare system and patients.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge