Ziqiao Weng
Learning Topology-Aware Implicit Field for Unified Pulmonary Tree Modeling with Incomplete Topological Supervision
Feb 02, 2026Abstract:Pulmonary trees extracted from CT images frequently exhibit topological incompleteness, such as missing or disconnected branches, which substantially degrades downstream anatomical analysis and limits the applicability of existing pulmonary tree modeling pipelines. Current approaches typically rely on dense volumetric processing or explicit graph reasoning, leading to limited efficiency and reduced robustness under realistic structural corruption. We propose TopoField, a topology-aware implicit modeling framework that treats topology repair as a first-class modeling problem and enables unified multi-task inference for pulmonary tree analysis. TopoField represents pulmonary anatomy using sparse surface and skeleton point clouds and learns a continuous implicit field that supports topology repair without relying on complete or explicit disconnection annotations, by training on synthetically introduced structural disruptions over \textit{already} incomplete trees. Building upon the repaired implicit representation, anatomical labeling and lung segment reconstruction are jointly inferred through task-specific implicit functions within a single forward pass.Extensive experiments on the Lung3D+ dataset demonstrate that TopoField consistently improves topological completeness and achieves accurate anatomical labeling and lung segment reconstruction under challenging incomplete scenarios. Owing to its implicit formulation, TopoField attains high computational efficiency, completing all tasks in just over one second per case, highlighting its practicality for large-scale and time-sensitive clinical applications. Code and data will be available at https://github.com/HINTLab/TopoField.
HiFusion: Hierarchical Intra-Spot Alignment and Regional Context Fusion for Spatial Gene Expression Prediction from Histopathology
Nov 19, 2025Abstract:Spatial transcriptomics (ST) bridges gene expression and tissue morphology but faces clinical adoption barriers due to technical complexity and prohibitive costs. While computational methods predict gene expression from H&E-stained whole-slide images (WSIs), existing approaches often fail to capture the intricate biological heterogeneity within spots and are susceptible to morphological noise when integrating contextual information from surrounding tissue. To overcome these limitations, we propose HiFusion, a novel deep learning framework that integrates two complementary components. First, we introduce the Hierarchical Intra-Spot Modeling module that extracts fine-grained morphological representations through multi-resolution sub-patch decomposition, guided by a feature alignment loss to ensure semantic consistency across scales. Concurrently, we present the Context-aware Cross-scale Fusion module, which employs cross-attention to selectively incorporate biologically relevant regional context, thereby enhancing representational capacity. This architecture enables comprehensive modeling of both cellular-level features and tissue microenvironmental cues, which are essential for accurate gene expression prediction. Extensive experiments on two benchmark ST datasets demonstrate that HiFusion achieves state-of-the-art performance across both 2D slide-wise cross-validation and more challenging 3D sample-specific scenarios. These results underscore HiFusion's potential as a robust, accurate, and scalable solution for ST inference from routine histopathology.
PANORAMA: The Rise of Omnidirectional Vision in the Embodied AI Era
Sep 16, 2025Abstract:Omnidirectional vision, using 360-degree vision to understand the environment, has become increasingly critical across domains like robotics, industrial inspection, and environmental monitoring. Compared to traditional pinhole vision, omnidirectional vision provides holistic environmental awareness, significantly enhancing the completeness of scene perception and the reliability of decision-making. However, foundational research in this area has historically lagged behind traditional pinhole vision. This talk presents an emerging trend in the embodied AI era: the rapid development of omnidirectional vision, driven by growing industrial demand and academic interest. We highlight recent breakthroughs in omnidirectional generation, omnidirectional perception, omnidirectional understanding, and related datasets. Drawing on insights from both academia and industry, we propose an ideal panoramic system architecture in the embodied AI era, PANORAMA, which consists of four key subsystems. Moreover, we offer in-depth opinions related to emerging trends and cross-community impacts at the intersection of panoramic vision and embodied AI, along with the future roadmap and open challenges. This overview synthesizes state-of-the-art advancements and outlines challenges and opportunities for future research in building robust, general-purpose omnidirectional AI systems in the embodied AI era.
Are Multimodal Large Language Models Ready for Omnidirectional Spatial Reasoning?
May 17, 2025Abstract:The 180x360 omnidirectional field of view captured by 360-degree cameras enables their use in a wide range of applications such as embodied AI and virtual reality. Although recent advances in multimodal large language models (MLLMs) have shown promise in visual-spatial reasoning, most studies focus on standard pinhole-view images, leaving omnidirectional perception largely unexplored. In this paper, we ask: Are MLLMs ready for omnidirectional spatial reasoning? To investigate this, we introduce OSR-Bench, the first benchmark specifically designed for this setting. OSR-Bench includes over 153,000 diverse question-answer pairs grounded in high-fidelity panoramic indoor scene maps. It covers key reasoning types including object counting, relative distance, and direction. We also propose a negative sampling strategy that inserts non-existent objects into prompts to evaluate hallucination and grounding robustness. For fine-grained analysis, we design a two-stage evaluation framework assessing both cognitive map generation and QA accuracy using rotation-invariant matching and a combination of rule-based and LLM-based metrics. We evaluate eight state-of-the-art MLLMs, including GPT-4o, Gemini 1.5 Pro, and leading open-source models under zero-shot settings. Results show that current models struggle with spatial reasoning in panoramic contexts, highlighting the need for more perceptually grounded MLLMs. OSR-Bench and code will be released at: https://huggingface.co/datasets/UUUserna/OSR-Bench
Efficient 4D fMRI ASD Classification using Spatial-Temporal-Omics-based Learning Framework
Feb 26, 2025


Abstract:Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder impacting social and behavioral development. Resting-state fMRI, a non-invasive tool for capturing brain connectivity patterns, aids in early ASD diagnosis and differentiation from typical controls (TC). However, previous methods, which rely on either mean time series or full 4D data, are limited by a lack of spatial information or by high computational costs. This underscores the need for an efficient solution that preserves both spatial and temporal information. In this paper, we propose a novel, simple, and efficient spatial-temporal-omics learning framework designed to efficiently extract spatio-temporal features from fMRI for ASD classification. Our approach addresses these limitations by utilizing 3D time-domain derivatives as the spatial-temporal inter-voxel omics, which preserve full spatial resolution while capturing diverse statistical characteristics of the time series at each voxel. Meanwhile, functional connectivity features serve as the spatial-temporal inter-regional omics, capturing correlations across brain regions. Extensive experiments and ablation studies on the ABIDE dataset demonstrate that our framework significantly outperforms previous methods while maintaining computational efficiency. We believe our research offers valuable insights that will inform and advance future ASD studies, particularly in the realm of spatial-temporal-omics-based learning.
Efficient Anatomical Labeling of Pulmonary Tree Structures via Implicit Point-Graph Networks
Oct 05, 2023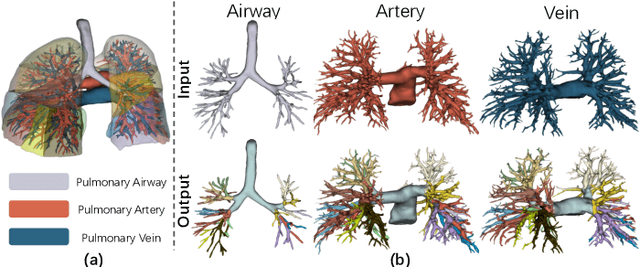
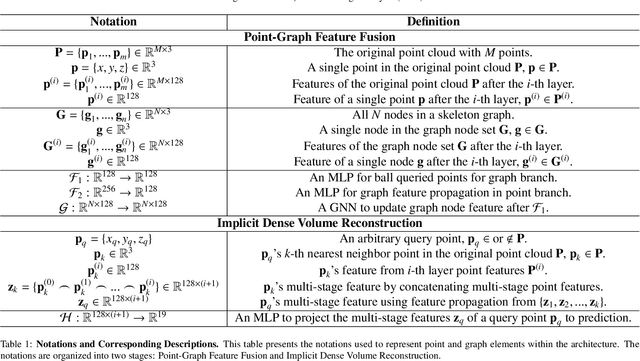
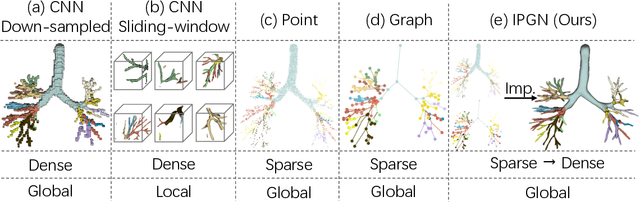
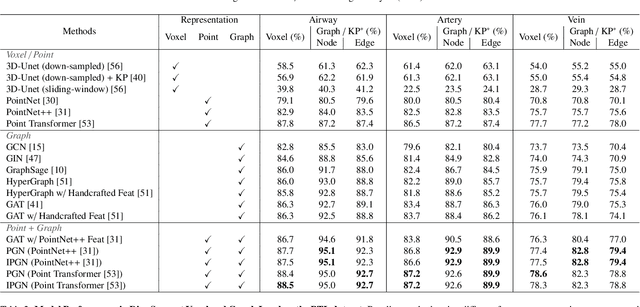
Abstract:Pulmonary diseases rank prominently among the principal causes of death worldwide. Curing them will require, among other things, a better understanding of the many complex 3D tree-shaped structures within the pulmonary system, such as airways, arteries, and veins. In theory, they can be modeled using high-resolution image stacks. Unfortunately, standard CNN approaches operating on dense voxel grids are prohibitively expensive. To remedy this, we introduce a point-based approach that preserves graph connectivity of tree skeleton and incorporates an implicit surface representation. It delivers SOTA accuracy at a low computational cost and the resulting models have usable surfaces. Due to the scarcity of publicly accessible data, we have also curated an extensive dataset to evaluate our approach and will make it public.
Topology Repairing of Disconnected Pulmonary Airways and Vessels: Baselines and a Dataset
Jun 28, 2023Abstract:Accurate segmentation of pulmonary airways and vessels is crucial for the diagnosis and treatment of pulmonary diseases. However, current deep learning approaches suffer from disconnectivity issues that hinder their clinical usefulness. To address this challenge, we propose a post-processing approach that leverages a data-driven method to repair the topology of disconnected pulmonary tubular structures. Our approach formulates the problem as a keypoint detection task, where a neural network is trained to predict keypoints that can bridge disconnected components. We use a training data synthesis pipeline that generates disconnected data from complete pulmonary structures. Moreover, the new Pulmonary Tree Repairing (PTR) dataset is publicly available, which comprises 800 complete 3D models of pulmonary airways, arteries, and veins, as well as the synthetic disconnected data. Our code and data are available at https://github.com/M3DV/pulmonary-tree-repairing.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge