Yoshitaka Inoue
GraphPINE: Graph Importance Propagation for Interpretable Drug Response Prediction
Apr 07, 2025



Abstract:Explainability is necessary for many tasks in biomedical research. Recent explainability methods have focused on attention, gradient, and Shapley value. These do not handle data with strong associated prior knowledge and fail to constrain explainability results based on known relationships between predictive features. We propose GraphPINE, a graph neural network (GNN) architecture leveraging domain-specific prior knowledge to initialize node importance optimized during training for drug response prediction. Typically, a manual post-prediction step examines literature (i.e., prior knowledge) to understand returned predictive features. While node importance can be obtained for gradient and attention after prediction, node importance from these methods lacks complementary prior knowledge; GraphPINE seeks to overcome this limitation. GraphPINE differs from other GNN gating methods by utilizing an LSTM-like sequential format. We introduce an importance propagation layer that unifies 1) updates for feature matrix and node importance and 2) uses GNN-based graph propagation of feature values. This initialization and updating mechanism allows for informed feature learning and improved graph representation. We apply GraphPINE to cancer drug response prediction using drug screening and gene data collected for over 5,000 gene nodes included in a gene-gene graph with a drug-target interaction (DTI) graph for initial importance. The gene-gene graph and DTIs were obtained from curated sources and weighted by article count discussing relationships between drugs and genes. GraphPINE achieves a PR-AUC of 0.894 and ROC-AUC of 0.796 across 952 drugs. Code is available at https://anonymous.4open.science/r/GraphPINE-40DE.
Protein-Mamba: Biological Mamba Models for Protein Function Prediction
Sep 22, 2024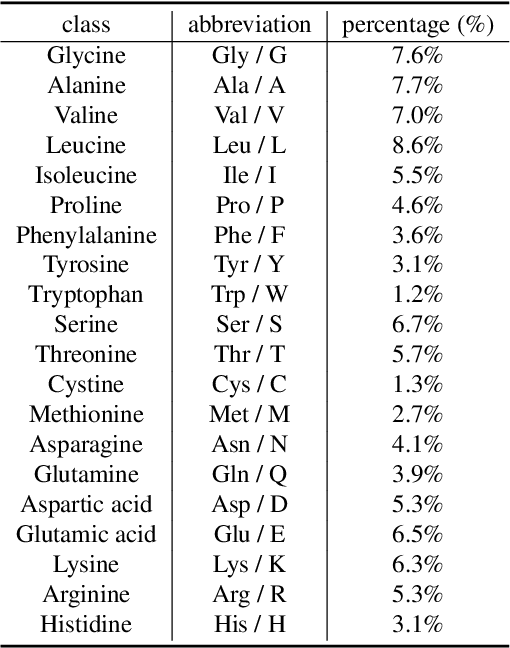


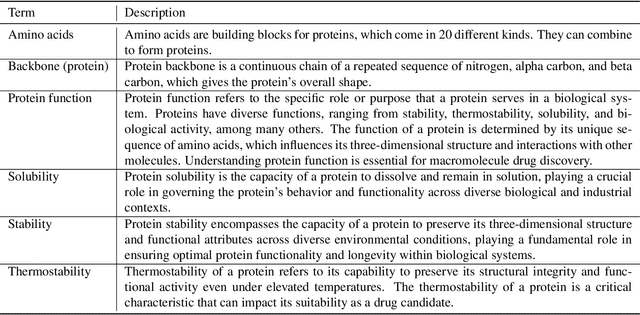
Abstract:Protein function prediction is a pivotal task in drug discovery, significantly impacting the development of effective and safe therapeutics. Traditional machine learning models often struggle with the complexity and variability inherent in predicting protein functions, necessitating more sophisticated approaches. In this work, we introduce Protein-Mamba, a novel two-stage model that leverages both self-supervised learning and fine-tuning to improve protein function prediction. The pre-training stage allows the model to capture general chemical structures and relationships from large, unlabeled datasets, while the fine-tuning stage refines these insights using specific labeled datasets, resulting in superior prediction performance. Our extensive experiments demonstrate that Protein-Mamba achieves competitive performance, compared with a couple of state-of-the-art methods across a range of protein function datasets. This model's ability to effectively utilize both unlabeled and labeled data highlights the potential of self-supervised learning in advancing protein function prediction and offers a promising direction for future research in drug discovery.
DrugAgent: Explainable Drug Repurposing Agent with Large Language Model-based Reasoning
Aug 23, 2024Abstract:Drug repurposing offers a promising avenue for accelerating drug development by identifying new therapeutic potentials of existing drugs. In this paper, we propose a multi-agent framework to enhance the drug repurposing process using state-of-the-art machine learning techniques and knowledge integration. Our framework comprises several specialized agents: an AI Agent trains robust drug-target interaction (DTI) models; a Knowledge Graph Agent utilizes the drug-gene interaction database (DGIdb), DrugBank, Comparative Toxicogenomics Database (CTD), and Search Tool for Interactions of Chemicals (STITCH) to systematically extract DTIs; and a Search Agent interacts with biomedical literature to annotate and verify computational predictions. By integrating outputs from these agents, our system effectively harnesses diverse data sources, including external databases, to propose viable repurposing candidates. Preliminary results demonstrate the potential of our approach in not only predicting drug-disease interactions but also in reducing the time and cost associated with traditional drug discovery methods. This paper highlights the scalability of multi-agent systems in biomedical research and their role in driving innovation in drug repurposing. Our approach not only outperforms existing methods in predicting drug repurposing potential but also provides interpretable results, paving the way for more efficient and cost-effective drug discovery processes.
Table Transformers for Imputing Textual Attributes
Aug 04, 2024Abstract:Missing data in tabular dataset is a common issue as the performance of downstream tasks usually depends on the completeness of the training dataset. Previous missing data imputation methods focus on numeric and categorical columns, but we propose a novel end-to-end approach called Table Transformers for Imputing Textual Attributes (TTITA) based on the transformer to impute unstructured textual columns using other columns in the table. We conduct extensive experiments on two Amazon Reviews datasets, and our approach shows competitive performance outperforming baseline models such as recurrent neural networks and Llama2. The performance improvement is more significant when the target sequence has a longer length. Additionally, we incorporated multi-task learning to simultaneously impute for heterogeneous columns, boosting the performance for text imputation. We also qualitatively compare with ChatGPT for realistic applications.
drGAT: Attention-Guided Gene Assessment of Drug Response Utilizing a Drug-Cell-Gene Heterogeneous Network
May 14, 2024



Abstract:Drug development is a lengthy process with a high failure rate. Increasingly, machine learning is utilized to facilitate the drug development processes. These models aim to enhance our understanding of drug characteristics, including their activity in biological contexts. However, a major challenge in drug response (DR) prediction is model interpretability as it aids in the validation of findings. This is important in biomedicine, where models need to be understandable in comparison with established knowledge of drug interactions with proteins. drGAT, a graph deep learning model, leverages a heterogeneous graph composed of relationships between proteins, cell lines, and drugs. drGAT is designed with two objectives: DR prediction as a binary sensitivity prediction and elucidation of drug mechanism from attention coefficients. drGAT has demonstrated superior performance over existing models, achieving 78\% accuracy (and precision), and 76\% F1 score for 269 DNA-damaging compounds of the NCI60 drug response dataset. To assess the model's interpretability, we conducted a review of drug-gene co-occurrences in Pubmed abstracts in comparison to the top 5 genes with the highest attention coefficients for each drug. We also examined whether known relationships were retained in the model by inspecting the neighborhoods of topoisomerase-related drugs. For example, our model retained TOP1 as a highly weighted predictive feature for irinotecan and topotecan, in addition to other genes that could potentially be regulators of the drugs. Our method can be used to accurately predict sensitivity to drugs and may be useful in the identification of biomarkers relating to the treatment of cancer patients.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge