Xuehui Shi
A Concept-based Interpretable Model for the Diagnosis of Choroid Neoplasias using Multimodal Data
Mar 08, 2024Abstract:Diagnosing rare diseases presents a common challenge in clinical practice, necessitating the expertise of specialists for accurate identification. The advent of machine learning offers a promising solution, while the development of such technologies is hindered by the scarcity of data on rare conditions and the demand for models that are both interpretable and trustworthy in a clinical context. Interpretable AI, with its capacity for human-readable outputs, can facilitate validation by clinicians and contribute to medical education. In the current work, we focus on choroid neoplasias, the most prevalent form of eye cancer in adults, albeit rare with 5.1 per million. We built the so-far largest dataset consisting of 750 patients, incorporating three distinct imaging modalities collected from 2004 to 2022. Our work introduces a concept-based interpretable model that distinguishes between three types of choroidal tumors, integrating insights from domain experts via radiological reports. Remarkably, this model not only achieves an F1 score of 0.91, rivaling that of black-box models, but also boosts the diagnostic accuracy of junior doctors by 42%. This study highlights the significant potential of interpretable machine learning in improving the diagnosis of rare diseases, laying a groundwork for future breakthroughs in medical AI that could tackle a wider array of complex health scenarios.
VisionFM: a Multi-Modal Multi-Task Vision Foundation Model for Generalist Ophthalmic Artificial Intelligence
Oct 08, 2023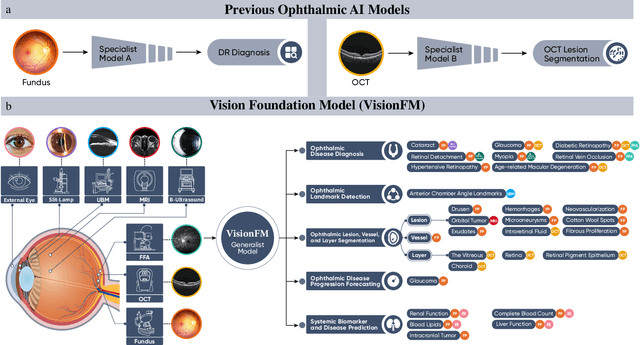
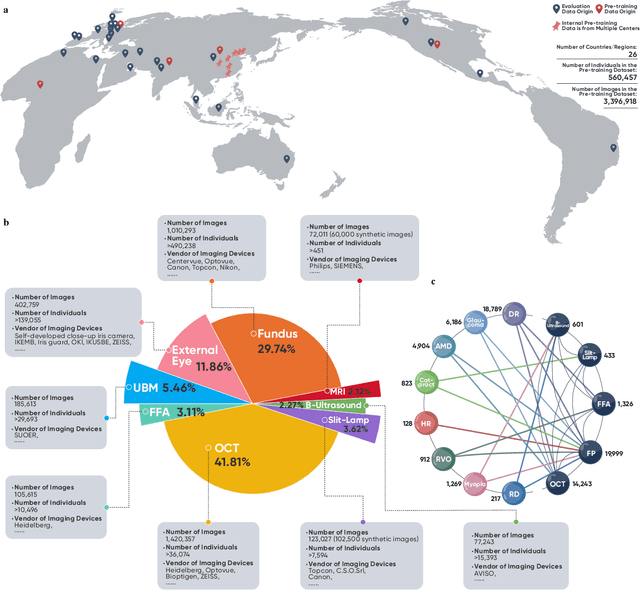

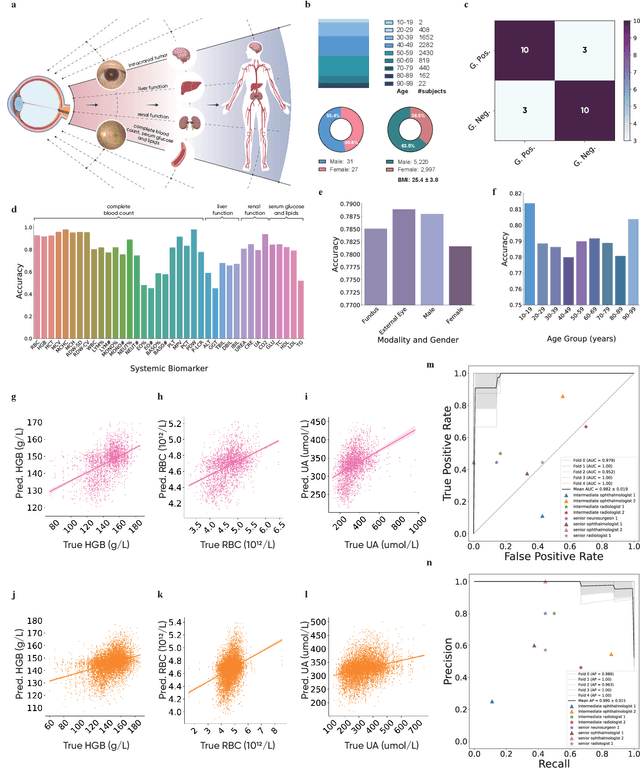
Abstract:We present VisionFM, a foundation model pre-trained with 3.4 million ophthalmic images from 560,457 individuals, covering a broad range of ophthalmic diseases, modalities, imaging devices, and demography. After pre-training, VisionFM provides a foundation to foster multiple ophthalmic artificial intelligence (AI) applications, such as disease screening and diagnosis, disease prognosis, subclassification of disease phenotype, and systemic biomarker and disease prediction, with each application enhanced with expert-level intelligence and accuracy. The generalist intelligence of VisionFM outperformed ophthalmologists with basic and intermediate levels in jointly diagnosing 12 common ophthalmic diseases. Evaluated on a new large-scale ophthalmic disease diagnosis benchmark database, as well as a new large-scale segmentation and detection benchmark database, VisionFM outperformed strong baseline deep neural networks. The ophthalmic image representations learned by VisionFM exhibited noteworthy explainability, and demonstrated strong generalizability to new ophthalmic modalities, disease spectrum, and imaging devices. As a foundation model, VisionFM has a large capacity to learn from diverse ophthalmic imaging data and disparate datasets. To be commensurate with this capacity, in addition to the real data used for pre-training, we also generated and leveraged synthetic ophthalmic imaging data. Experimental results revealed that synthetic data that passed visual Turing tests, can also enhance the representation learning capability of VisionFM, leading to substantial performance gains on downstream ophthalmic AI tasks. Beyond the ophthalmic AI applications developed, validated, and demonstrated in this work, substantial further applications can be achieved in an efficient and cost-effective manner using VisionFM as the foundation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge