Weidong He
Retinal OCT Synthesis with Denoising Diffusion Probabilistic Models for Layer Segmentation
Nov 09, 2023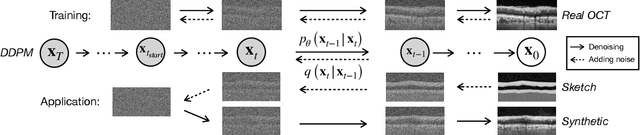
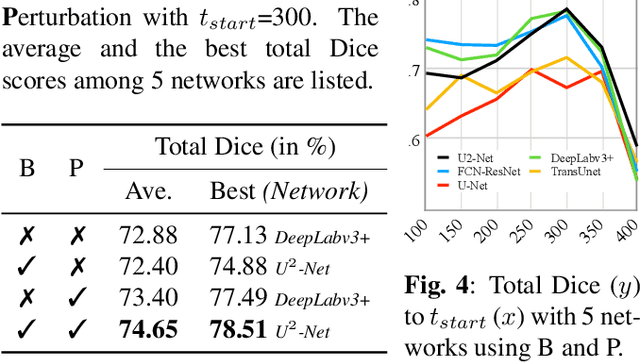
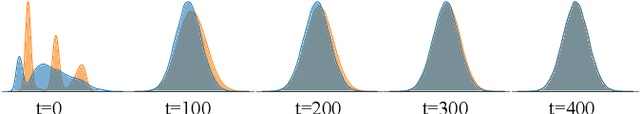
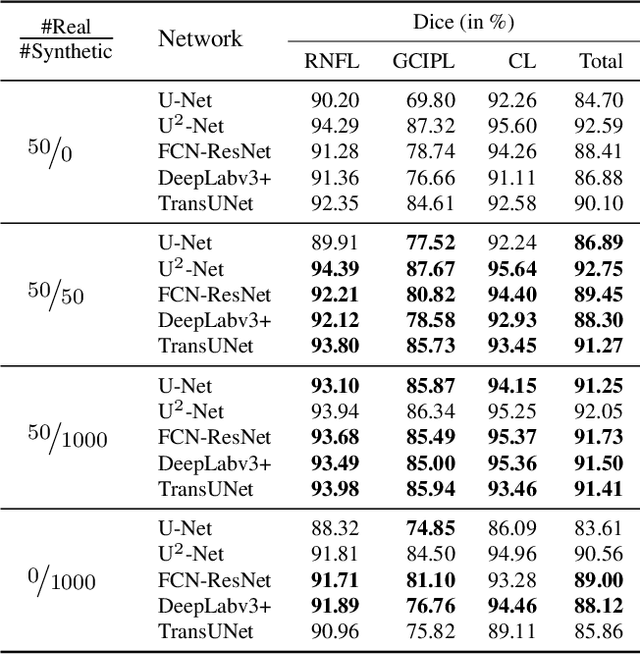
Abstract:Modern biomedical image analysis using deep learning often encounters the challenge of limited annotated data. To overcome this issue, deep generative models can be employed to synthesize realistic biomedical images. In this regard, we propose an image synthesis method that utilizes denoising diffusion probabilistic models (DDPMs) to automatically generate retinal optical coherence tomography (OCT) images. By providing rough layer sketches, the trained DDPMs can generate realistic circumpapillary OCT images. We further find that more accurate pseudo labels can be obtained through knowledge adaptation, which greatly benefits the segmentation task. Through this, we observe a consistent improvement in layer segmentation accuracy, which is validated using various neural networks. Furthermore, we have discovered that a layer segmentation model trained solely with synthesized images can achieve comparable results to a model trained exclusively with real images. These findings demonstrate the promising potential of DDPMs in reducing the need for manual annotations of retinal OCT images.
Winning the CVPR'2022 AQTC Challenge: A Two-stage Function-centric Approach
Jun 22, 2022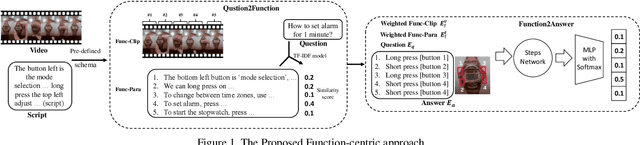
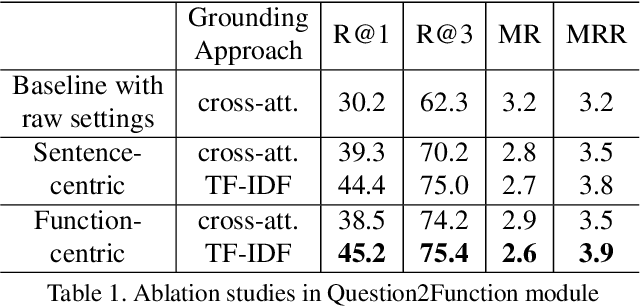
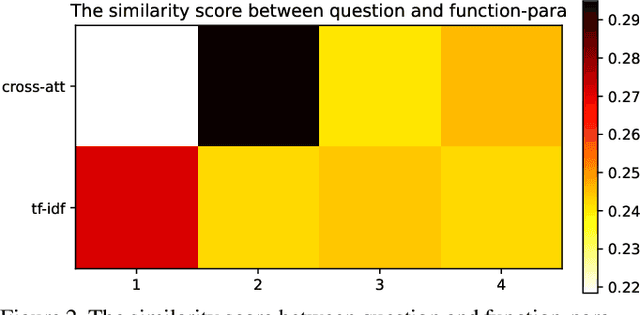
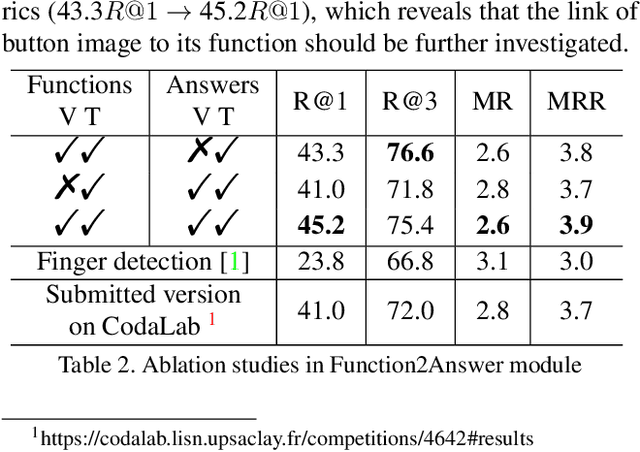
Abstract:Affordance-centric Question-driven Task Completion for Egocentric Assistant(AQTC) is a novel task which helps AI assistant learn from instructional videos and scripts and guide the user step-by-step. In this paper, we deal with the AQTC via a two-stage Function-centric approach, which consists of Question2Function Module to ground the question with the related function and Function2Answer Module to predict the action based on the historical steps. We evaluated several possible solutions in each module and obtained significant gains compared to the given baselines. Our code is available at \url{https://github.com/starsholic/LOVEU-CVPR22-AQTC}.
Clustering Bioactive Molecules in 3D Chemical Space with Unsupervised Deep Learning
Feb 09, 2019Abstract:Unsupervised clustering has broad applications in data stratification, pattern investigation and new discovery beyond existing knowledge. In particular, clustering of bioactive molecules facilitates chemical space mapping, structure-activity studies, and drug discovery. These tasks, conventionally conducted by similarity-based methods, are complicated by data complexity and diversity. We ex-plored the superior learning capability of deep autoencoders for unsupervised clustering of 1.39 mil-lion bioactive molecules into band-clusters in a 3-dimensional latent chemical space. These band-clusters, displayed by a space-navigation simulation software, band molecules of selected bioactivity classes into individual band-clusters possessing unique sets of common sub-structural features beyond structural similarity. These sub-structural features form the frameworks of the literature-reported pharmacophores and privileged fragments. Within each band-cluster, molecules are further banded into selected sub-regions with respect to their bioactivity target, sub-structural features and molecular scaffolds. Our method is potentially applicable for big data clustering tasks of different fields.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge