Vanessa Kyriakopoulou
School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, Department of Forensic and Neurodevelopmental Science, Institute of Psychiatry, Psychology & Neuroscience, King's College London, London, United Kingdom
Meta-learning Slice-to-Volume Reconstruction in Fetal Brain MRI using Implicit Neural Representations
May 14, 2025



Abstract:High-resolution slice-to-volume reconstruction (SVR) from multiple motion-corrupted low-resolution 2D slices constitutes a critical step in image-based diagnostics of moving subjects, such as fetal brain Magnetic Resonance Imaging (MRI). Existing solutions struggle with image artifacts and severe subject motion or require slice pre-alignment to achieve satisfying reconstruction performance. We propose a novel SVR method to enable fast and accurate MRI reconstruction even in cases of severe image and motion corruption. Our approach performs motion correction, outlier handling, and super-resolution reconstruction with all operations being entirely based on implicit neural representations. The model can be initialized with task-specific priors through fully self-supervised meta-learning on either simulated or real-world data. In extensive experiments including over 480 reconstructions of simulated and clinical MRI brain data from different centers, we prove the utility of our method in cases of severe subject motion and image artifacts. Our results demonstrate improvements in reconstruction quality, especially in the presence of severe motion, compared to state-of-the-art methods, and up to 50% reduction in reconstruction time.
Automatic 8-tissue Segmentation for 6-month Infant Brains
Aug 27, 2024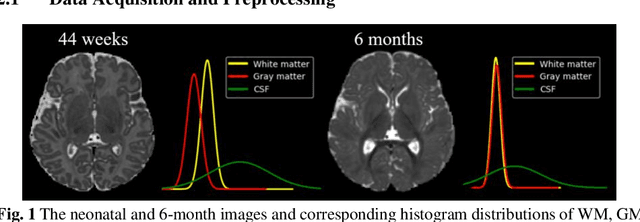
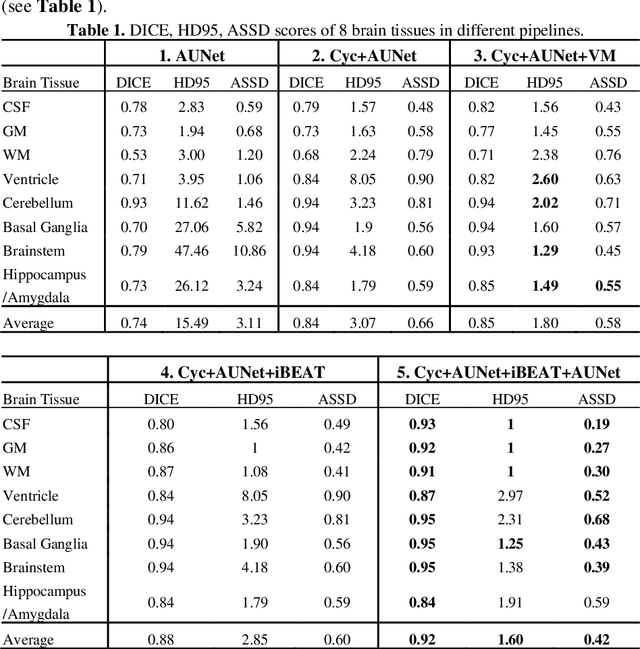
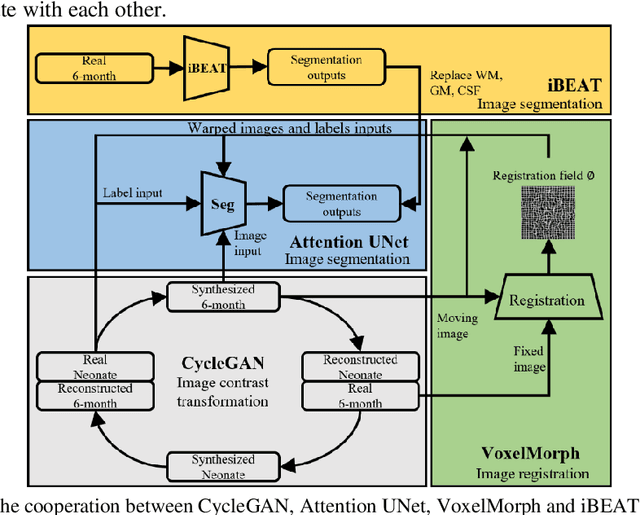
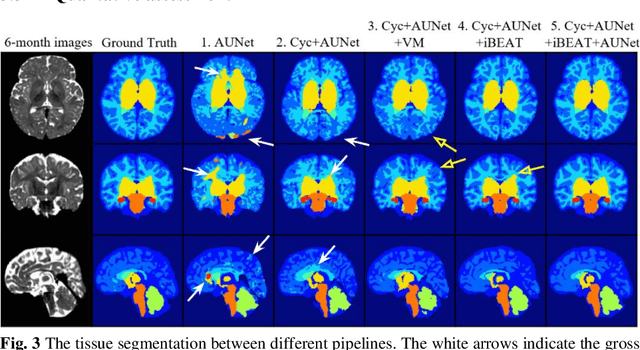
Abstract:Numerous studies have highlighted that atypical brain development, particularly during infancy and toddlerhood, is linked to an increased likelihood of being diagnosed with a neurodevelopmental condition, such as autism. Accurate brain tissue segmentations for morphological analysis are essential in numerous infant studies. However, due to ongoing white matter (WM) myelination changing tissue contrast in T1- and T2-weighted images, automatic tissue segmentation in 6-month infants is particularly difficult. On the other hand, manual labelling by experts is time-consuming and labor-intensive. In this study, we propose the first 8-tissue segmentation pipeline for six-month-old infant brains. This pipeline utilizes domain adaptation (DA) techniques to leverage our longitudinal data, including neonatal images segmented with the neonatal Developing Human Connectome Project structural pipeline. Our pipeline takes raw 6-month images as inputs and generates the 8-tissue segmentation as outputs, forming an end-to-end segmentation pipeline. The segmented tissues include WM, gray matter (GM), cerebrospinal fluid (CSF), ventricles, cerebellum, basal ganglia, brainstem, and hippocampus/amygdala. Cycle-Consistent Generative Adversarial Network (CycleGAN) and Attention U-Net were employed to achieve the image contrast transformation between neonatal and 6-month images and perform tissue segmentation on the synthesized 6-month images (neonatal images with 6-month intensity contrast), respectively. Moreover, we incorporated the segmentation outputs from Infant Brain Extraction and Analysis Toolbox (iBEAT) and another Attention U-Net to further enhance the performance and construct the end-to-end segmentation pipeline. Our evaluation with real 6-month images achieved a DICE score of 0.92, an HD95 of 1.6, and an ASSD of 0.42.
CINA: Conditional Implicit Neural Atlas for Spatio-Temporal Representation of Fetal Brains
Mar 13, 2024



Abstract:We introduce a conditional implicit neural atlas (CINA) for spatio-temporal atlas generation from Magnetic Resonance Images (MRI) of the neurotypical and pathological fetal brain, that is fully independent of affine or non-rigid registration. During training, CINA learns a general representation of the fetal brain and encodes subject specific information into latent code. After training, CINA can construct a faithful atlas with tissue probability maps of the fetal brain for any gestational age (GA) and anatomical variation covered within the training domain. Thus, CINA is competent to represent both, neurotypical and pathological brains. Furthermore, a trained CINA model can be fit to brain MRI of unseen subjects via test-time optimization of the latent code. CINA can then produce probabilistic tissue maps tailored to a particular subject. We evaluate our method on a total of 198 T2 weighted MRI of normal and abnormal fetal brains from the dHCP and FeTA datasets. We demonstrate CINA's capability to represent a fetal brain atlas that can be flexibly conditioned on GA and on anatomical variations like ventricular volume or degree of cortical folding, making it a suitable tool for modeling both neurotypical and pathological brains. We quantify the fidelity of our atlas by means of tissue segmentation and age prediction and compare it to an established baseline. CINA demonstrates superior accuracy for neurotypical brains and pathological brains with ventriculomegaly. Moreover, CINA scores a mean absolute error of 0.23 weeks in fetal brain age prediction, further confirming an accurate representation of fetal brain development.
Conditional Temporal Attention Networks for Neonatal Cortical Surface Reconstruction
Jul 21, 2023Abstract:Cortical surface reconstruction plays a fundamental role in modeling the rapid brain development during the perinatal period. In this work, we propose Conditional Temporal Attention Network (CoTAN), a fast end-to-end framework for diffeomorphic neonatal cortical surface reconstruction. CoTAN predicts multi-resolution stationary velocity fields (SVF) from neonatal brain magnetic resonance images (MRI). Instead of integrating multiple SVFs, CoTAN introduces attention mechanisms to learn a conditional time-varying velocity field (CTVF) by computing the weighted sum of all SVFs at each integration step. The importance of each SVF, which is estimated by learned attention maps, is conditioned on the age of the neonates and varies with the time step of integration. The proposed CTVF defines a diffeomorphic surface deformation, which reduces mesh self-intersection errors effectively. It only requires 0.21 seconds to deform an initial template mesh to cortical white matter and pial surfaces for each brain hemisphere. CoTAN is validated on the Developing Human Connectome Project (dHCP) dataset with 877 3D brain MR images acquired from preterm and term born neonates. Compared to state-of-the-art baselines, CoTAN achieves superior performance with only 0.12mm geometric error and 0.07% self-intersecting faces. The visualization of our attention maps illustrates that CoTAN indeed learns coarse-to-fine surface deformations automatically without intermediate supervision.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge