Maria Deprez
School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom
Automatic 8-tissue Segmentation for 6-month Infant Brains
Aug 27, 2024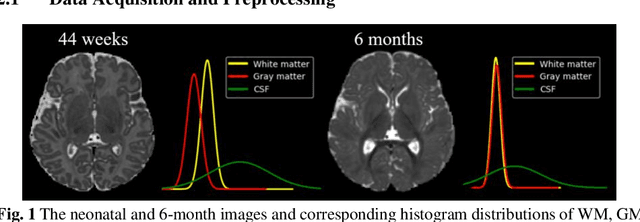
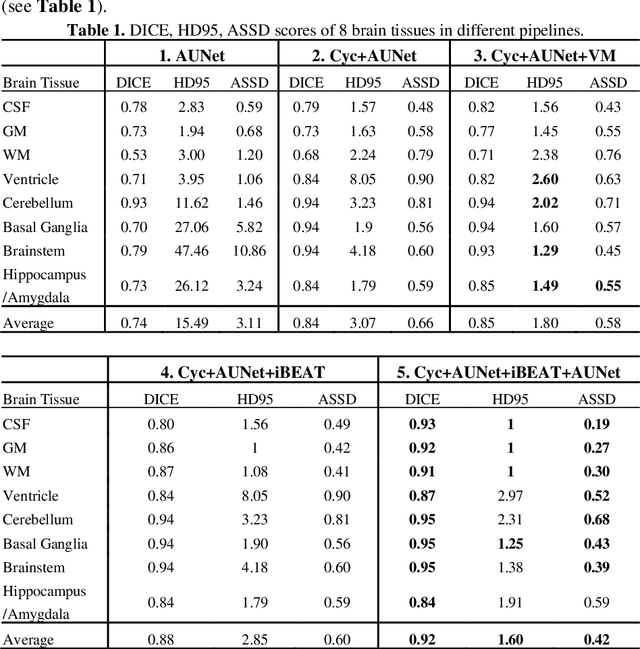
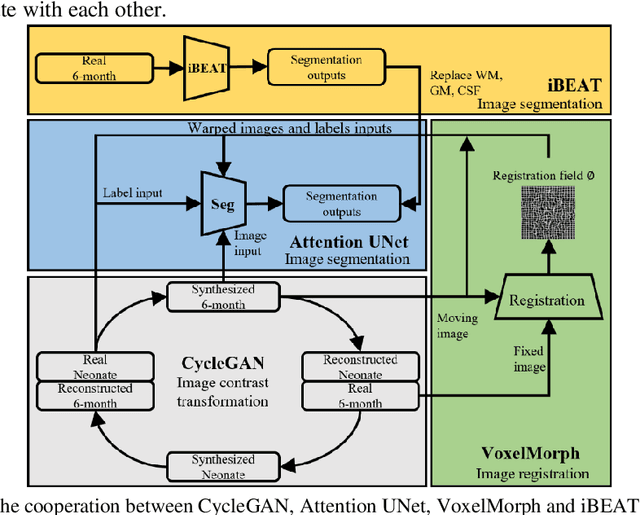
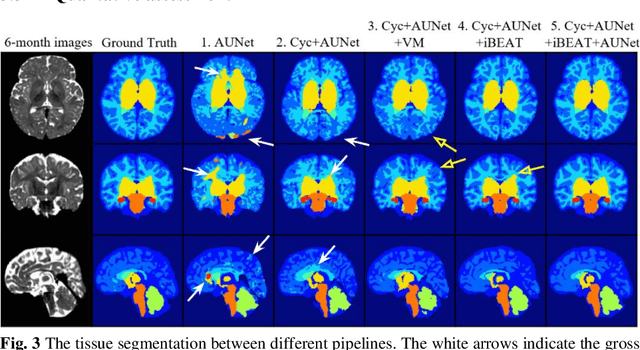
Abstract:Numerous studies have highlighted that atypical brain development, particularly during infancy and toddlerhood, is linked to an increased likelihood of being diagnosed with a neurodevelopmental condition, such as autism. Accurate brain tissue segmentations for morphological analysis are essential in numerous infant studies. However, due to ongoing white matter (WM) myelination changing tissue contrast in T1- and T2-weighted images, automatic tissue segmentation in 6-month infants is particularly difficult. On the other hand, manual labelling by experts is time-consuming and labor-intensive. In this study, we propose the first 8-tissue segmentation pipeline for six-month-old infant brains. This pipeline utilizes domain adaptation (DA) techniques to leverage our longitudinal data, including neonatal images segmented with the neonatal Developing Human Connectome Project structural pipeline. Our pipeline takes raw 6-month images as inputs and generates the 8-tissue segmentation as outputs, forming an end-to-end segmentation pipeline. The segmented tissues include WM, gray matter (GM), cerebrospinal fluid (CSF), ventricles, cerebellum, basal ganglia, brainstem, and hippocampus/amygdala. Cycle-Consistent Generative Adversarial Network (CycleGAN) and Attention U-Net were employed to achieve the image contrast transformation between neonatal and 6-month images and perform tissue segmentation on the synthesized 6-month images (neonatal images with 6-month intensity contrast), respectively. Moreover, we incorporated the segmentation outputs from Infant Brain Extraction and Analysis Toolbox (iBEAT) and another Attention U-Net to further enhance the performance and construct the end-to-end segmentation pipeline. Our evaluation with real 6-month images achieved a DICE score of 0.92, an HD95 of 1.6, and an ASSD of 0.42.
Multi-task learning for joint weakly-supervised segmentation and aortic arch anomaly classification in fetal cardiac MRI
Nov 13, 2023Abstract:Congenital Heart Disease (CHD) is a group of cardiac malformations present already during fetal life, representing the prevailing category of birth defects globally. Our aim in this study is to aid 3D fetal vessel topology visualisation in aortic arch anomalies, a group which encompasses a range of conditions with significant anatomical heterogeneity. We present a multi-task framework for automated multi-class fetal vessel segmentation from 3D black blood T2w MRI and anomaly classification. Our training data consists of binary manual segmentation masks of the cardiac vessels' region in individual subjects and fully-labelled anomaly-specific population atlases. Our framework combines deep learning label propagation using VoxelMorph with 3D Attention U-Net segmentation and DenseNet121 anomaly classification. We target 11 cardiac vessels and three distinct aortic arch anomalies, including double aortic arch, right aortic arch, and suspected coarctation of the aorta. We incorporate an anomaly classifier into our segmentation pipeline, delivering a multi-task framework with the primary motivation of correcting topological inaccuracies of the segmentation. The hypothesis is that the multi-task approach will encourage the segmenter network to learn anomaly-specific features. As a secondary motivation, an automated diagnosis tool may have the potential to enhance diagnostic confidence in a decision support setting. Our results showcase that our proposed training strategy significantly outperforms label propagation and a network trained exclusively on propagated labels. Our classifier outperforms a classifier trained exclusively on T2w volume images, with an average balanced accuracy of 0.99 (0.01) after joint training. Adding a classifier improves the anatomical and topological accuracy of all correctly classified double aortic arch subjects.
* Accepted for publication at the Journal of Machine Learning for Biomedical Imaging (MELBA) https://melba-journal.org/2023:015
An automated pipeline for quantitative T2* fetal body MRI and segmentation at low field
Aug 09, 2023Abstract:Fetal Magnetic Resonance Imaging at low field strengths is emerging as an exciting direction in perinatal health. Clinical low field (0.55T) scanners are beneficial for fetal imaging due to their reduced susceptibility-induced artefacts, increased T2* values, and wider bore (widening access for the increasingly obese pregnant population). However, the lack of standard automated image processing tools such as segmentation and reconstruction hampers wider clinical use. In this study, we introduce a semi-automatic pipeline using quantitative MRI for the fetal body at low field strength resulting in fast and detailed quantitative T2* relaxometry analysis of all major fetal body organs. Multi-echo dynamic sequences of the fetal body were acquired and reconstructed into a single high-resolution volume using deformable slice-to-volume reconstruction, generating both structural and quantitative T2* 3D volumes. A neural network trained using a semi-supervised approach was created to automatically segment these fetal body 3D volumes into ten different organs (resulting in dice values > 0.74 for 8 out of 10 organs). The T2* values revealed a strong relationship with GA in the lungs, liver, and kidney parenchyma (R^2>0.5). This pipeline was used successfully for a wide range of GAs (17-40 weeks), and is robust to motion artefacts. Low field fetal MRI can be used to perform advanced MRI analysis, and is a viable option for clinical scanning.
Fetal MRI by robust deep generative prior reconstruction and diffeomorphic registration: application to gestational age prediction
Oct 29, 2021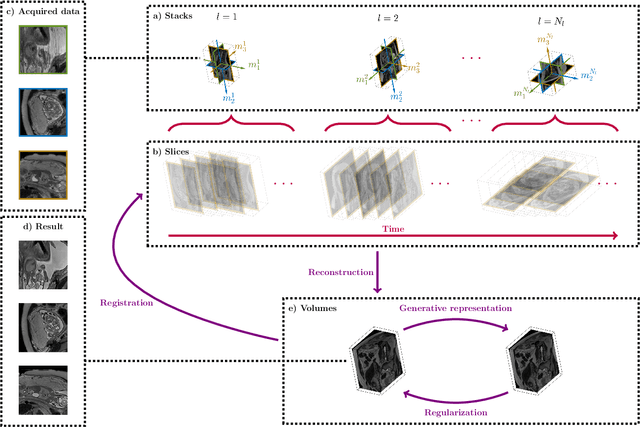
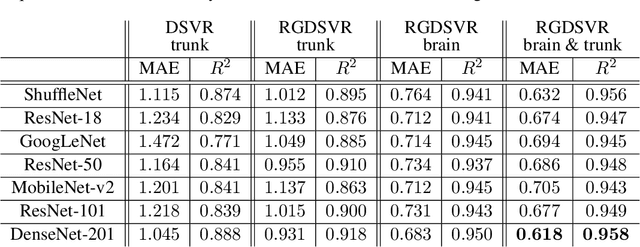

Abstract:Magnetic resonance imaging of whole fetal body and placenta is limited by different sources of motion affecting the womb. Usual scanning techniques employ single-shot multi-slice sequences where anatomical information in different slices may be subject to different deformations, contrast variations or artifacts. Volumetric reconstruction formulations have been proposed to correct for these factors, but they must accommodate a non-homogeneous and non-isotropic sampling, so regularization becomes necessary. Thus, in this paper we propose a deep generative prior for robust volumetric reconstructions integrated with a diffeomorphic volume to slice registration method. Experiments are performed to validate our contributions and compare with a state of the art method in a cohort of $72$ fetal datasets in the range of $20-36$ weeks gestational age. Results suggest improved image resolution and more accurate prediction of gestational age at scan when comparing to a state of the art reconstruction method. In addition, gestational age prediction results from our volumetric reconstructions compare favourably with existing brain-based approaches, with boosted accuracy when integrating information of organs other than the brain. Namely, a mean absolute error of $0.618$ weeks ($R^2=0.958$) is achieved when combining fetal brain and trunk information.
Self-supervised Recurrent Neural Network for 4D Abdominal and In-utero MR Imaging
Aug 28, 2019



Abstract:Accurately estimating and correcting the motion artifacts are crucial for 3D image reconstruction of the abdominal and in-utero magnetic resonance imaging (MRI). The state-of-art methods are based on slice-to-volume registration (SVR) where multiple 2D image stacks are acquired in three orthogonal orientations. In this work, we present a novel reconstruction pipeline that only needs one orientation of 2D MRI scans and can reconstruct the full high-resolution image without masking or registration steps. The framework consists of two main stages: the respiratory motion estimation using a self-supervised recurrent neural network, which learns the respiratory signals that are naturally embedded in the asymmetry relationship of the neighborhood slices and cluster them according to a respiratory state. Then, we train a 3D deconvolutional network for super-resolution (SR) reconstruction of the sparsely selected 2D images using integrated reconstruction and total variation loss. We evaluate the classification accuracy on 5 simulated images and compare our results with the SVR method in adult abdominal and in-utero MRI scans. The results show that the proposed pipeline can accurately estimate the respiratory state and reconstruct 4D SR volumes with better or similar performance to the 3D SVR pipeline with less than 20\% sparsely selected slices. The method has great potential to transform the 4D abdominal and in-utero MRI in clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge