Sungwon Lee
Universal Lymph Node Detection in Multiparametric MRI with Selective Augmentation
Apr 07, 2025Abstract:Robust localization of lymph nodes (LNs) in multiparametric MRI (mpMRI) is critical for the assessment of lymphadenopathy. Radiologists routinely measure the size of LN to distinguish benign from malignant nodes, which would require subsequent cancer staging. Sizing is a cumbersome task compounded by the diverse appearances of LNs in mpMRI, which renders their measurement difficult. Furthermore, smaller and potentially metastatic LNs could be missed during a busy clinical day. To alleviate these imaging and workflow problems, we propose a pipeline to universally detect both benign and metastatic nodes in the body for their ensuing measurement. The recently proposed VFNet neural network was employed to identify LN in T2 fat suppressed and diffusion weighted imaging (DWI) sequences acquired by various scanners with a variety of exam protocols. We also use a selective augmentation technique known as Intra-Label LISA (ILL) to diversify the input data samples the model sees during training, such that it improves its robustness during the evaluation phase. We achieved a sensitivity of $\sim$83\% with ILL vs. $\sim$80\% without ILL at 4 FP/vol. Compared with current LN detection approaches evaluated on mpMRI, we show a sensitivity improvement of $\sim$9\% at 4 FP/vol.
Graph-Based Small Bowel Path Tracking with Cylindrical Constraints
Jul 29, 2022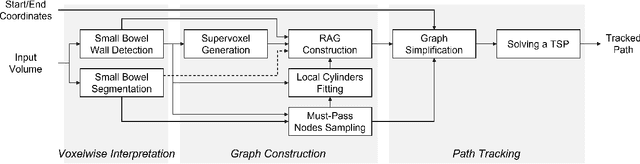
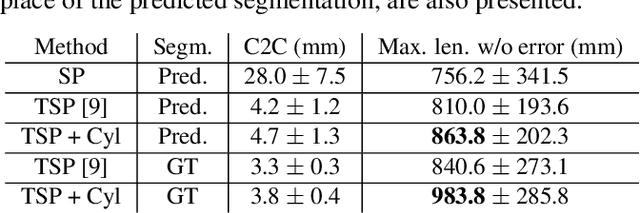
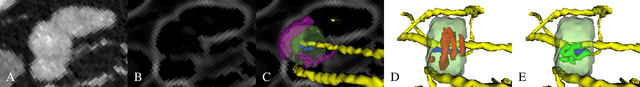
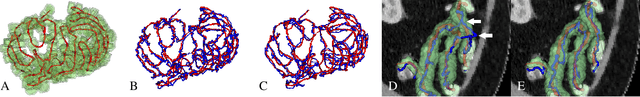
Abstract:We present a new graph-based method for small bowel path tracking based on cylindrical constraints. A distinctive characteristic of the small bowel compared to other organs is the contact between parts of itself along its course, which makes the path tracking difficult together with the indistinct appearance of the wall. It causes the tracked path to easily cross over the walls when relying on low-level features like the wall detection. To circumvent this, a series of cylinders that are fitted along the course of the small bowel are used to guide the tracking to more reliable directions. It is implemented as soft constraints using a new cost function. The proposed method is evaluated against ground-truth paths that are all connected from start to end of the small bowel for 10 abdominal CT scans. The proposed method showed clear improvements compared to the baseline method in tracking the path without making an error. Improvements of 6.6% and 17.0%, in terms of the tracked length, were observed for two different settings related to the small bowel segmentation.
Universal Lymph Node Detection in T2 MRI using Neural Networks
Mar 31, 2022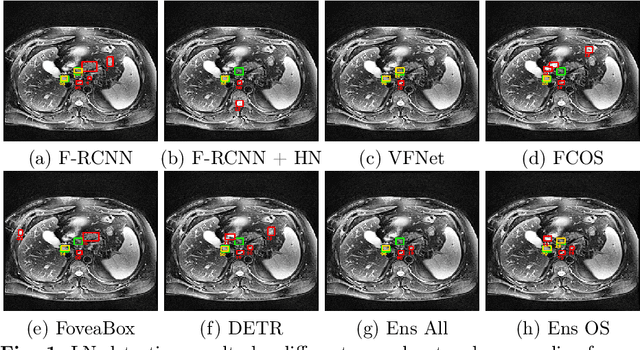
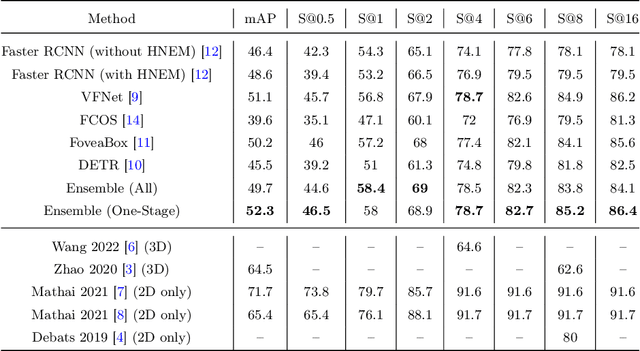
Abstract:Purpose: Identification of abdominal Lymph Nodes (LN) that are suspicious for metastasis in T2 Magnetic Resonance Imaging (MRI) scans is critical for staging of lymphoproliferative diseases. Prior work on LN detection has been limited to specific anatomical regions of the body (pelvis, rectum) in single MR slices. Therefore, the development of a universal approach to detect LN in full T2 MRI volumes is highly desirable. Methods: In this study, a Computer Aided Detection (CAD) pipeline to universally identify abdominal LN in volumetric T2 MRI using neural networks is proposed. First, we trained various neural network models for detecting LN: Faster RCNN with and without Hard Negative Example Mining (HNEM), FCOS, FoveaBox, VFNet, and Detection Transformer (DETR). Next, we show that the state-of-the-art (SOTA) VFNet model with Adaptive Training Sample Selection (ATSS) outperforms Faster RCNN with HNEM. Finally, we ensembled models that surpassed a 45% mAP threshold. We found that the VFNet model and one-stage model ensemble can be interchangeably used in the CAD pipeline. Results: Experiments on 122 test T2 MRI volumes revealed that VFNet achieved a 51.1% mAP and 78.7% recall at 4 false positives (FP) per volume, while the one-stage model ensemble achieved a mAP of 52.3% and sensitivity of 78.7% at 4FP. Conclusion: Our contribution is a CAD pipeline that detects LN in T2 MRI volumes, resulting in a sensitivity improvement of $\sim$14 points over the current SOTA method for LN detection (sensitivity of 78.7% at 4 FP vs. 64.6% at 5 FP per volume).
Lymph Node Detection in T2 MRI with Transformers
Nov 09, 2021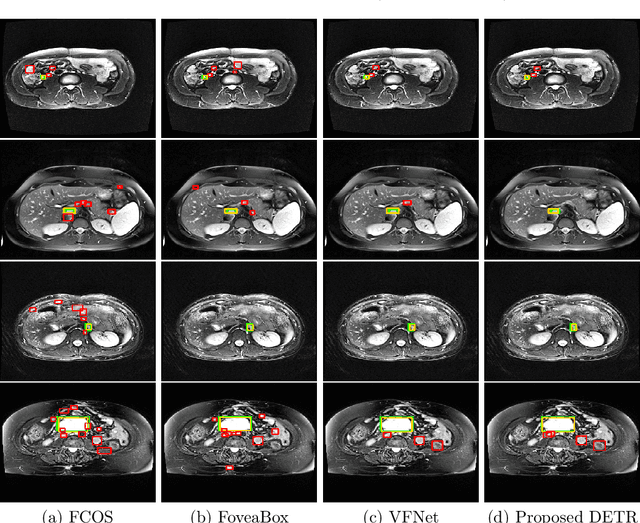
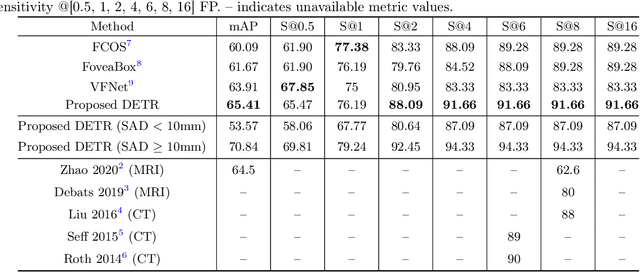
Abstract:Identification of lymph nodes (LN) in T2 Magnetic Resonance Imaging (MRI) is an important step performed by radiologists during the assessment of lymphoproliferative diseases. The size of the nodes play a crucial role in their staging, and radiologists sometimes use an additional contrast sequence such as diffusion weighted imaging (DWI) for confirmation. However, lymph nodes have diverse appearances in T2 MRI scans, making it tough to stage for metastasis. Furthermore, radiologists often miss smaller metastatic lymph nodes over the course of a busy day. To deal with these issues, we propose to use the DEtection TRansformer (DETR) network to localize suspicious metastatic lymph nodes for staging in challenging T2 MRI scans acquired by different scanners and exam protocols. False positives (FP) were reduced through a bounding box fusion technique, and a precision of 65.41\% and sensitivity of 91.66\% at 4 FP per image was achieved. To the best of our knowledge, our results improve upon the current state-of-the-art for lymph node detection in T2 MRI scans.
A Graph-theoretic Algorithm for Small Bowel Path Tracking in CT Scans
Oct 01, 2021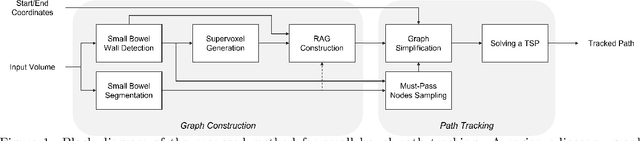

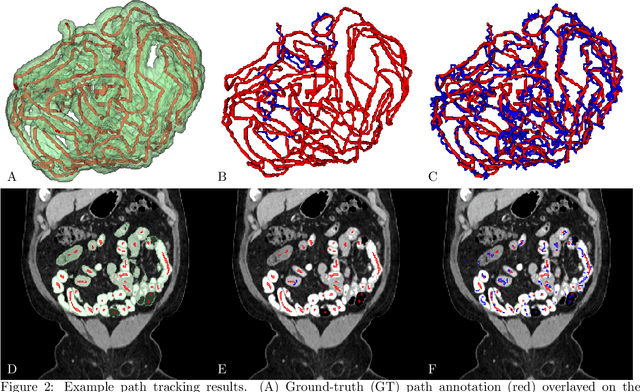
Abstract:We present a novel graph-theoretic method for small bowel path tracking. It is formulated as finding the minimum cost path between given start and end nodes on a graph that is constructed based on the bowel wall detection. We observed that a trivial solution with many short-cuts is easily made even with the wall detection, where the tracked path penetrates indistinct walls around the contact between different parts of the small bowel. Thus, we propose to include must-pass nodes in finding the path to better cover the entire course of the small bowel. The proposed method does not entail training with ground-truth paths while the previous methods do. We acquired ground-truth paths that are all connected from start to end of the small bowel for 10 abdominal CT scans, which enables the evaluation of the path tracking for the entire course of the small bowel. The proposed method showed clear improvements in terms of several metrics compared to the baseline method. The maximum length of the path that is tracked without an error per scan, by the proposed method, is above 800mm on average.
Unsupervised Domain Adaptation for Small Bowel Segmentation using Disentangled Representation
Jul 06, 2021Abstract:We present a novel unsupervised domain adaptation method for small bowel segmentation based on feature disentanglement. To make the domain adaptation more controllable, we disentangle intensity and non-intensity features within a unique two-stream auto-encoding architecture, and selectively adapt the non-intensity features that are believed to be more transferable across domains. The segmentation prediction is performed by aggregating the disentangled features. We evaluated our method using intravenous contrast-enhanced abdominal CT scans with and without oral contrast, which are used as source and target domains, respectively. The proposed method showed clear improvements in terms of three different metrics compared to other domain adaptation methods that are without the feature disentanglement. The method brings small bowel segmentation closer to clinical application.
Deep Small Bowel Segmentation with Cylindrical Topological Constraints
Jul 16, 2020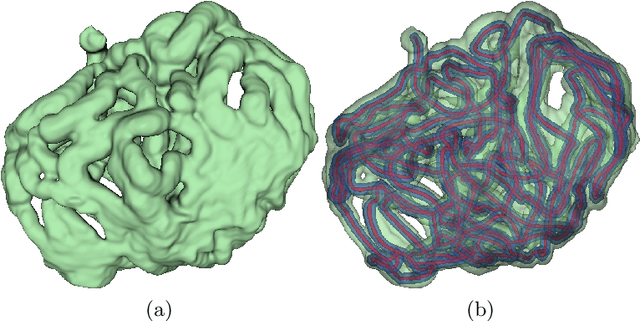
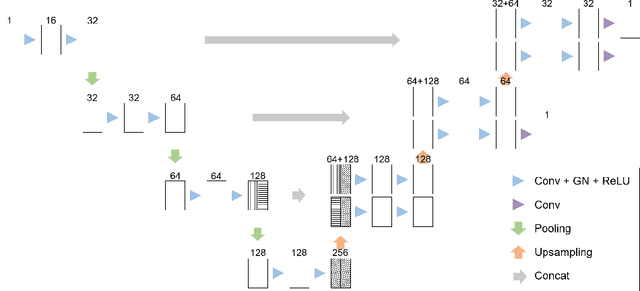
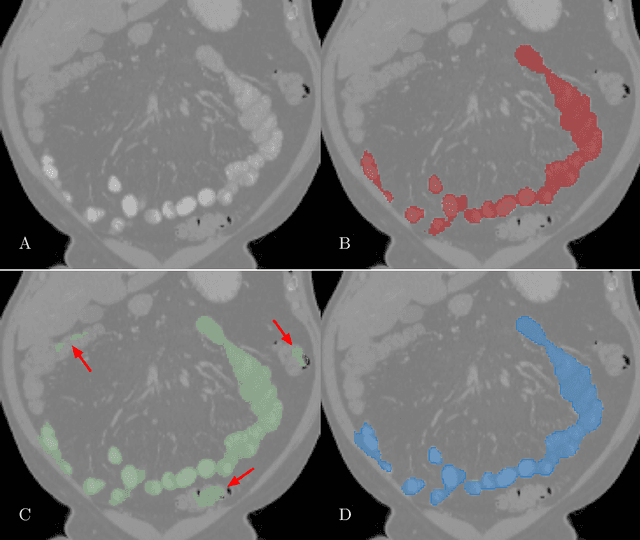
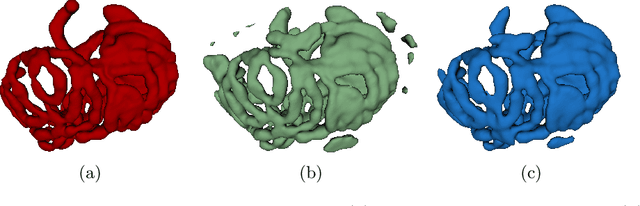
Abstract:We present a novel method for small bowel segmentation where a cylindrical topological constraint based on persistent homology is applied. To address the touching issue which could break the applied constraint, we propose to augment a network with an additional branch to predict an inner cylinder of the small bowel. Since the inner cylinder is free of the touching issue, a cylindrical shape constraint applied on this augmented branch guides the network to generate a topologically correct segmentation. For strict evaluation, we achieved an abdominal computed tomography dataset with dense segmentation ground-truths. The proposed method showed clear improvements in terms of four different metrics compared to the baseline method, and also showed the statistical significance from a paired t-test.
Cross-Domain Medical Image Translation by Shared Latent Gaussian Mixture Model
Jul 14, 2020



Abstract:Current deep learning based segmentation models often generalize poorly between domains due to insufficient training data. In real-world clinical applications, cross-domain image analysis tools are in high demand since medical images from different domains are often needed to achieve a precise diagnosis. An important example in radiology is generalizing from non-contrast CT to contrast enhanced CTs. Contrast enhanced CT scans at different phases are used to enhance certain pathologies or organs. Many existing cross-domain image-to-image translation models have been shown to improve cross-domain segmentation of large organs. However, such models lack the ability to preserve fine structures during the translation process, which is significant for many clinical applications, such as segmenting small calcified plaques in the aorta and pelvic arteries. In order to preserve fine structures during medical image translation, we propose a patch-based model using shared latent variables from a Gaussian mixture model. We compare our image translation framework to several state-of-the-art methods on cross-domain image translation and show our model does a better job preserving fine structures. The superior performance of our model is verified by performing two tasks with the translated images - detection and segmentation of aortic plaques and pancreas segmentation. We expect the utility of our framework will extend to other problems beyond segmentation due to the improved quality of the generated images and enhanced ability to preserve small structures.
COVID-19-CT-CXR: a freely accessible and weakly labeled chest X-ray and CT image collection on COVID-19 from biomedical literature
Jun 11, 2020



Abstract:The latest threat to global health is the COVID-19 outbreak. Although there exist large datasets of chest X-rays (CXR) and computed tomography (CT) scans, few COVID-19 image collections are currently available due to patient privacy. At the same time, there is a rapid growth of COVID-19-relevant articles in the biomedical literature. Here, we present COVID-19-CT-CXR, a public database of COVID-19 CXR and CT images, which are automatically extracted from COVID-19-relevant articles from the PubMed Central Open Access (PMC-OA) Subset. We extracted figures, associated captions, and relevant figure descriptions in the article and separated compound figures into subfigures. We also designed a deep-learning model to distinguish them from other figure types and to classify them accordingly. The final database includes 1,327 CT and 263 CXR images (as of May 9, 2020) with their relevant text. To demonstrate the utility of COVID-19-CT-CXR, we conducted four case studies. (1) We show that COVID-19-CT-CXR, when used as additional training data, is able to contribute to improved DL performance for the classification of COVID-19 and non-COVID-19 CT. (2) We collected CT images of influenza and trained a DL baseline to distinguish a diagnosis of COVID-19, influenza, or normal or other types of diseases on CT. (3) We trained an unsupervised one-class classifier from non-COVID-19 CXR and performed anomaly detection to detect COVID-19 CXR. (4) From text-mined captions and figure descriptions, we compared clinical symptoms and clinical findings of COVID-19 vs. those of influenza to demonstrate the disease differences in the scientific publications. We believe that our work is complementary to existing resources and hope that it will contribute to medical image analysis of the COVID-19 pandemic. The dataset, code, and DL models are publicly available at https://github.com/ncbi-nlp/COVID-19-CT-CXR.
Image Translation by Latent Union of Subspaces for Cross-Domain Plaque Detection
May 22, 2020

Abstract:Calcified plaque in the aorta and pelvic arteries is associated with coronary artery calcification and is a strong predictor of heart attack. Current calcified plaque detection models show poor generalizability to different domains (ie. pre-contrast vs. post-contrast CT scans). Many recent works have shown how cross domain object detection can be improved using an image translation model which translates between domains using a single shared latent space. However, while current image translation models do a good job preserving global/intermediate level structures they often have trouble preserving tiny structures. In medical imaging applications, preserving small structures is important since these structures can carry information which is highly relevant for disease diagnosis. Recent works on image reconstruction show that complex real-world images are better reconstructed using a union of subspaces approach. Since small image patches are used to train the image translation model, it makes sense to enforce that each patch be represented by a linear combination of subspaces which may correspond to the different parts of the body present in that patch. Motivated by this, we propose an image translation network using a shared union of subspaces constraint and show our approach preserves subtle structures (plaques) better than the conventional method. We further applied our method to a cross domain plaque detection task and show significant improvement compared to the state-of-the art method.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge