Steve Nyemba
Distillation to Enhance the Portability of Risk Models Across Institutions with Large Patient Claims Database
Jul 06, 2022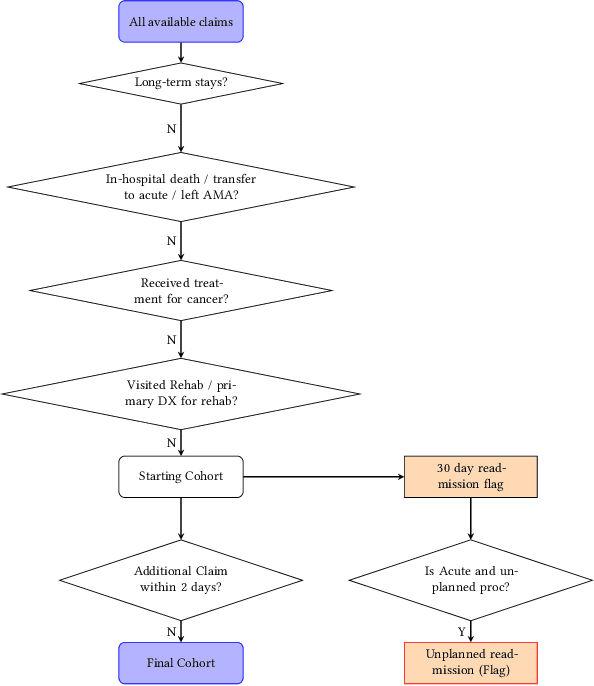
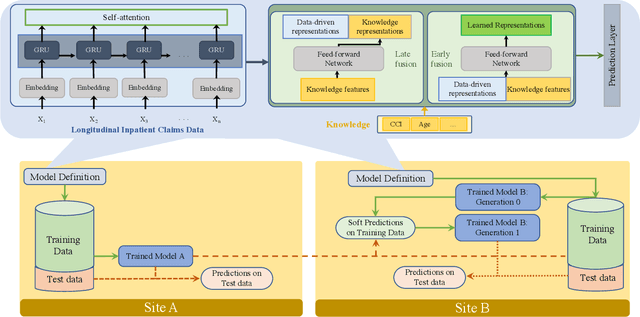

Abstract:Artificial intelligence, and particularly machine learning (ML), is increasingly developed and deployed to support healthcare in a variety of settings. However, clinical decision support (CDS) technologies based on ML need to be portable if they are to be adopted on a broad scale. In this respect, models developed at one institution should be reusable at another. Yet there are numerous examples of portability failure, particularly due to naive application of ML models. Portability failure can lead to suboptimal care and medical errors, which ultimately could prevent the adoption of ML-based CDS in practice. One specific healthcare challenge that could benefit from enhanced portability is the prediction of 30-day readmission risk. Research to date has shown that deep learning models can be effective at modeling such risk. In this work, we investigate the practicality of model portability through a cross-site evaluation of readmission prediction models. To do so, we apply a recurrent neural network, augmented with self-attention and blended with expert features, to build readmission prediction models for two independent large scale claims datasets. We further present a novel transfer learning technique that adapts the well-known method of born-again network (BAN) training. Our experiments show that direct application of ML models trained at one institution and tested at another institution perform worse than models trained and tested at the same institution. We further show that the transfer learning approach based on the BAN produces models that are better than those trained on just a single institution's data. Notably, this improvement is consistent across both sites and occurs after a single retraining, which illustrates the potential for a cheap and general model transfer mechanism of readmission risk prediction.
Blending Knowledge in Deep Recurrent Networks for Adverse Event Prediction at Hospital Discharge
Apr 09, 2021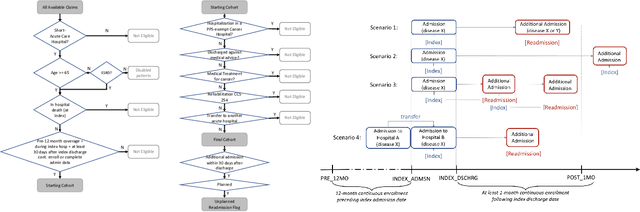
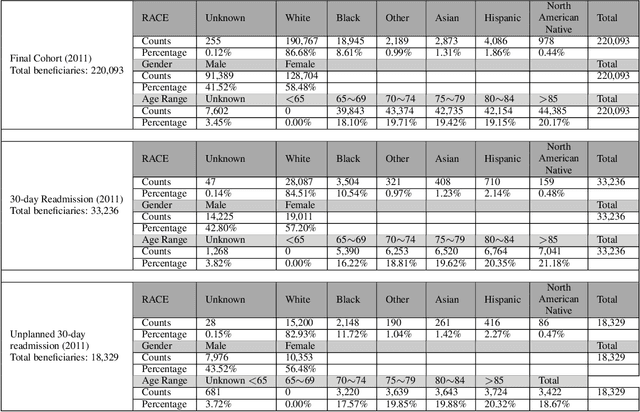
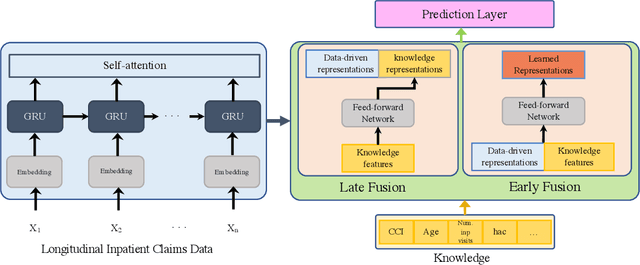

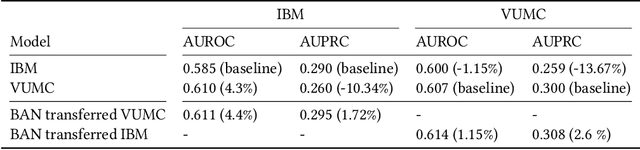
Abstract:Deep learning architectures have an extremely high-capacity for modeling complex data in a wide variety of domains. However, these architectures have been limited in their ability to support complex prediction problems using insurance claims data, such as readmission at 30 days, mainly due to data sparsity issue. Consequently, classical machine learning methods, especially those that embed domain knowledge in handcrafted features, are often on par with, and sometimes outperform, deep learning approaches. In this paper, we illustrate how the potential of deep learning can be achieved by blending domain knowledge within deep learning architectures to predict adverse events at hospital discharge, including readmissions. More specifically, we introduce a learning architecture that fuses a representation of patient data computed by a self-attention based recurrent neural network, with clinically relevant features. We conduct extensive experiments on a large claims dataset and show that the blended method outperforms the standard machine learning approaches.
Generating Electronic Health Records with Multiple Data Types and Constraints
Mar 23, 2020



Abstract:Sharing electronic health records (EHRs) on a large scale may lead to privacy intrusions. Recent research has shown that risks may be mitigated by simulating EHRs through generative adversarial network (GAN) frameworks. Yet the methods developed to date are limited because they 1) focus on generating data of a single type (e.g., diagnosis codes), neglecting other data types (e.g., demographics, procedures or vital signs) and 2) do not represent constraints between features. In this paper, we introduce a method to simulate EHRs composed of multiple data types by 1) refining the GAN model, 2) accounting for feature constraints, and 3) incorporating key utility measures for such generation tasks. Our analysis with over $770,000$ EHRs from Vanderbilt University Medical Center demonstrates that the new model achieves higher performance in terms of retaining basic statistics, cross-feature correlations, latent structural properties, feature constraints and associated patterns from real data, without sacrificing privacy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge