Shaodong Cao
Deep learning-driven pulmonary arteries and veins segmentation reveals demography-associated pulmonary vasculature anatomy
Apr 11, 2024Abstract:Pulmonary artery-vein segmentation is crucial for diagnosing pulmonary diseases and surgical planning, and is traditionally achieved by Computed Tomography Pulmonary Angiography (CTPA). However, concerns regarding adverse health effects from contrast agents used in CTPA have constrained its clinical utility. In contrast, identifying arteries and veins using non-contrast CT, a conventional and low-cost clinical examination routine, has long been considered impossible. Here we propose a High-abundant Pulmonary Artery-vein Segmentation (HiPaS) framework achieving accurate artery-vein segmentation on both non-contrast CT and CTPA across various spatial resolutions. HiPaS first performs spatial normalization on raw CT scans via a super-resolution module, and then iteratively achieves segmentation results at different branch levels by utilizing the low-level vessel segmentation as a prior for high-level vessel segmentation. We trained and validated HiPaS on our established multi-centric dataset comprising 1,073 CT volumes with meticulous manual annotation. Both quantitative experiments and clinical evaluation demonstrated the superior performance of HiPaS, achieving a dice score of 91.8% and a sensitivity of 98.0%. Further experiments demonstrated the non-inferiority of HiPaS segmentation on non-contrast CT compared to segmentation on CTPA. Employing HiPaS, we have conducted an anatomical study of pulmonary vasculature on 10,613 participants in China (five sites), discovering a new association between pulmonary vessel abundance and sex and age: vessel abundance is significantly higher in females than in males, and slightly decreases with age, under the controlling of lung volumes (p < 0.0001). HiPaS realizing accurate artery-vein segmentation delineates a promising avenue for clinical diagnosis and understanding pulmonary physiology in a non-invasive manner.
VoxelAtlasGAN: 3D Left Ventricle Segmentation on Echocardiography with Atlas Guided Generation and Voxel-to-voxel Discrimination
Jun 10, 2018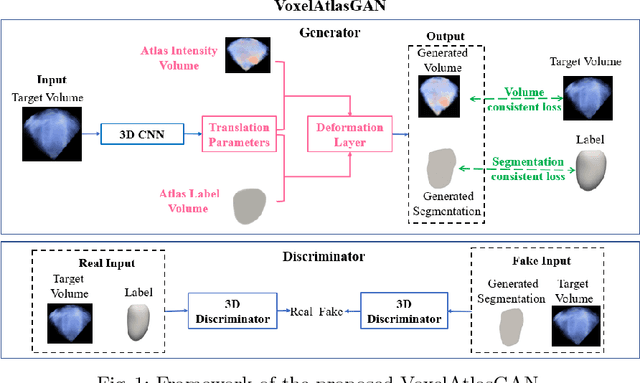
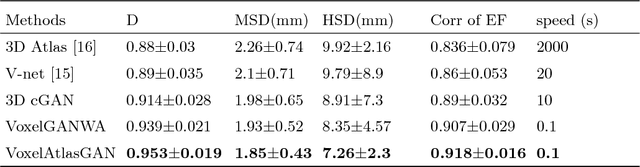
Abstract:3D left ventricle (LV) segmentation on echocardiography is very important for diagnosis and treatment of cardiac disease. It is not only because of that echocardiography is a real-time imaging technology and widespread in clinical application, but also because of that LV segmentation on 3D echocardiography can provide more full volume information of heart than LV segmentation on 2D echocardiography. However, 3D LV segmentation on echocardiography is still an open and challenging task owing to the lower contrast, higher noise and data dimensionality, limited annotation of 3D echocardiography. In this paper, we proposed a novel real-time framework, i.e., VoxelAtlasGAN, for 3D LV segmentation on 3D echocardiography. This framework has three contributions: 1) It is based on voxel-to-voxel conditional generative adversarial nets (cGAN). For the first time, cGAN is used for 3D LV segmentation on echocardiography. And cGAN advantageously fuses substantial 3D spatial context information from 3D echocardiography by self-learning structured loss; 2) For the first time, it embeds the atlas into an end-to-end optimization framework, which uses 3D LV atlas as a powerful prior knowledge to improve the inference speed, address the lower contrast and the limited annotation problems of 3D echocardiography; 3) It combines traditional discrimination loss and the new proposed consistent constraint, which further improves the generalization of the proposed framework. VoxelAtlasGAN was validated on 60 subjects on 3D echocardiography and it achieved satisfactory segmentation results and high inference speed. The mean surface distance is 1.85 mm, the mean hausdorff surface distance is 7.26 mm, mean dice is 0.953, the correlation of EF is 0.918, and the mean inference speed is 0.1s. These results have demonstrated that our proposed method has great potential for clinical application
Multi-views Fusion CNN for Left Ventricular Volumes Estimation on Cardiac MR Images
Apr 09, 2018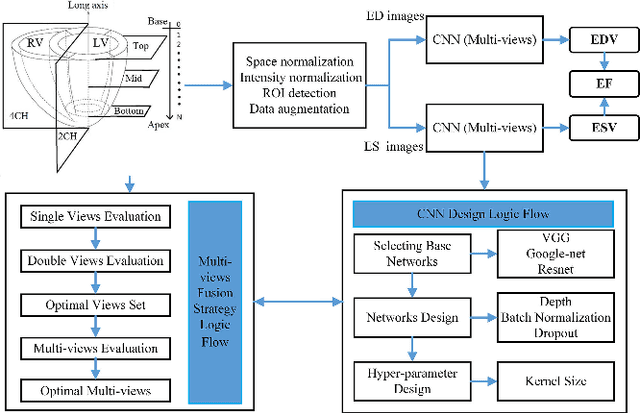
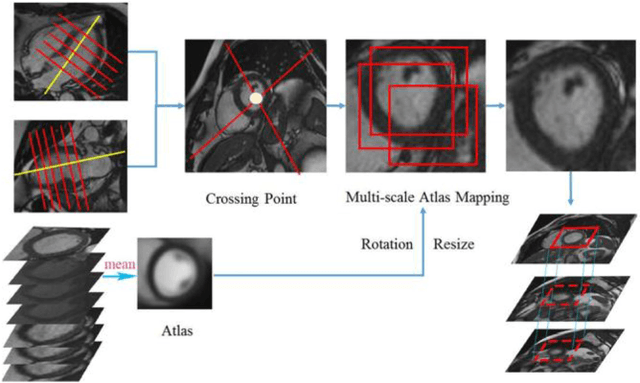
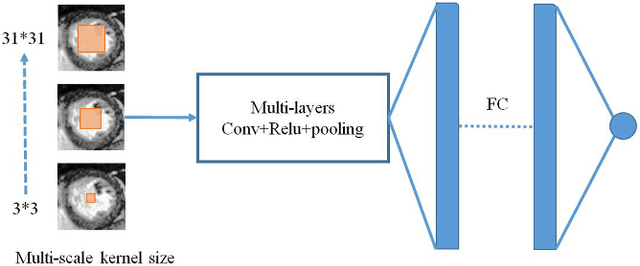
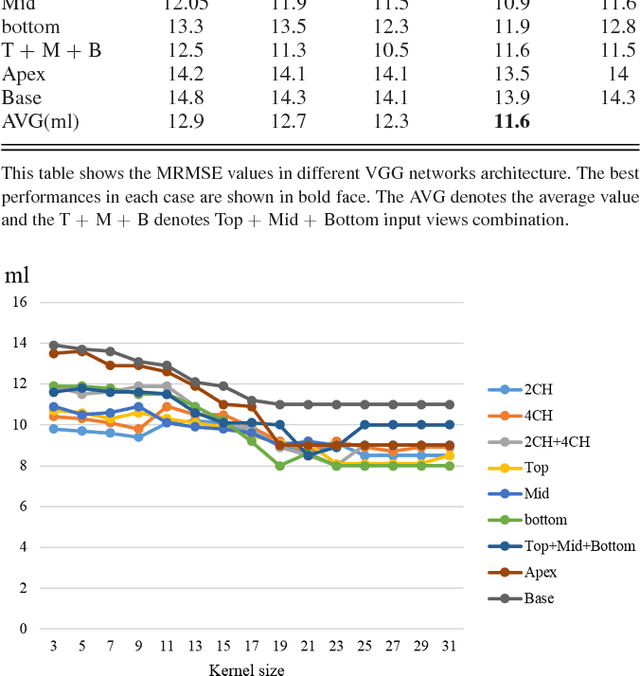
Abstract:Left ventricular (LV) volumes estimation is a critical procedure for cardiac disease diagnosis. The objective of this paper is to address direct LV volumes prediction task. Methods: In this paper, we propose a direct volumes prediction method based on the end-to-end deep convolutional neural networks (CNN). We study the end-to-end LV volumes prediction method in items of the data preprocessing, networks structure, and multi-views fusion strategy. The main contributions of this paper are the following aspects. First, we propose a new data preprocessing method on cardiac magnetic resonance (CMR). Second, we propose a new networks structure for end-to-end LV volumes estimation. Third, we explore the representational capacity of different slices, and propose a fusion strategy to improve the prediction accuracy. Results: The evaluation results show that the proposed method outperforms other state-of-the-art LV volumes estimation methods on the open accessible benchmark datasets. The clinical indexes derived from the predicted volumes agree well with the ground truth (EDV: R2=0.974, RMSE=9.6ml; ESV: R2=0.976, RMSE=7.1ml; EF: R2=0.828, RMSE =4.71%). Conclusion: Experimental results prove that the proposed method may be useful for LV volumes prediction task. Significance: The proposed method not only has application potential for cardiac diseases screening for large-scale CMR data, but also can be extended to other medical image research fields
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge