Scott L. Delp
Department of Biomedical Engineering, Stanford University, California, USA, Department of Orthopaedic Surgery, Stanford University, California, USA, Department of Mechanical Engineering, Stanford University, California, USA
Open source software for automatic subregional assessment of knee cartilage degradation using quantitative T2 relaxometry and deep learning
Dec 22, 2020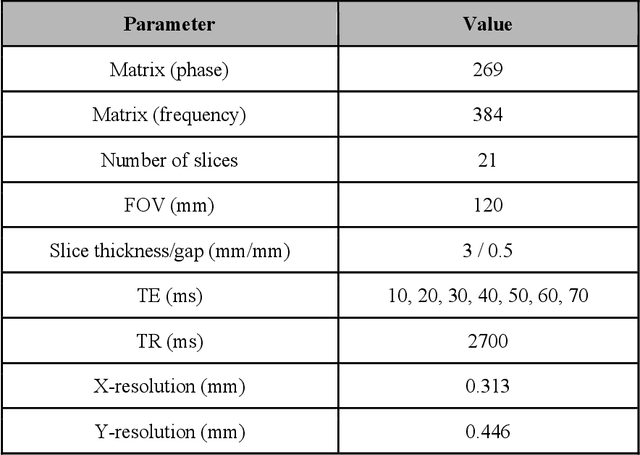
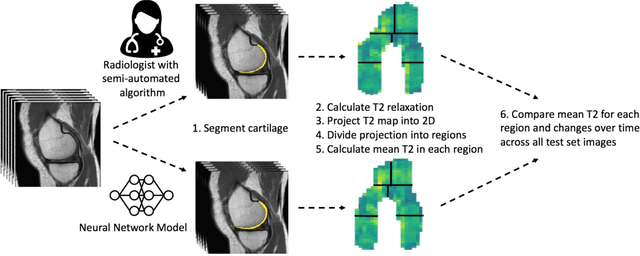
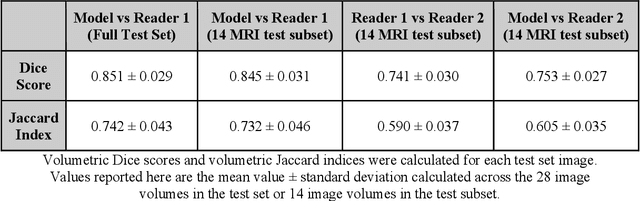
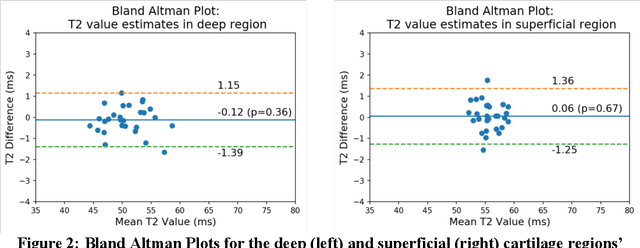
Abstract:Objective: We evaluate a fully-automated femoral cartilage segmentation model for measuring T2 relaxation values and longitudinal changes using multi-echo spin echo (MESE) MRI. We have open sourced this model and corresponding segmentations. Methods: We trained a neural network to segment femoral cartilage from MESE MRIs. Cartilage was divided into 12 subregions along medial-lateral, superficial-deep, and anterior-central-posterior boundaries. Subregional T2 values and four-year changes were calculated using a musculoskeletal radiologist's segmentations (Reader 1) and the model's segmentations. These were compared using 28 held out images. A subset of 14 images were also evaluated by a second expert (Reader 2) for comparison. Results: Model segmentations agreed with Reader 1 segmentations with a Dice score of 0.85 +/- 0.03. The model's estimated T2 values for individual subregions agreed with those of Reader 1 with an average Spearman correlation of 0.89 and average mean absolute error (MAE) of 1.34 ms. The model's estimated four-year change in T2 for individual regions agreed with Reader 1 with an average correlation of 0.80 and average MAE of 1.72 ms. The model agreed with Reader 1 at least as closely as Reader 2 agreed with Reader 1 in terms of Dice score (0.85 vs 0.75) and subregional T2 values. Conclusions: We present a fast, fully-automated model for segmentation of MESE MRIs. Assessments of cartilage health using its segmentations agree with those of an expert as closely as experts agree with one another. This has the potential to accelerate osteoarthritis research.
Learning to Run challenge: Synthesizing physiologically accurate motion using deep reinforcement learning
Mar 31, 2018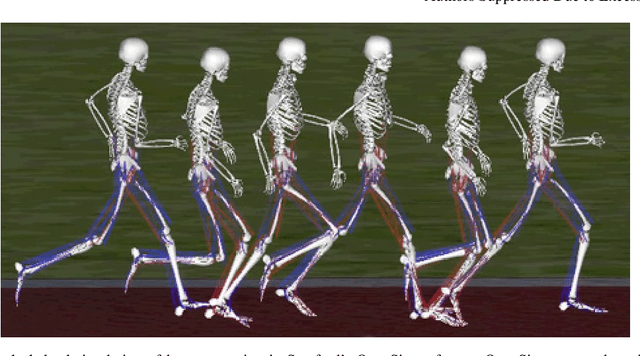
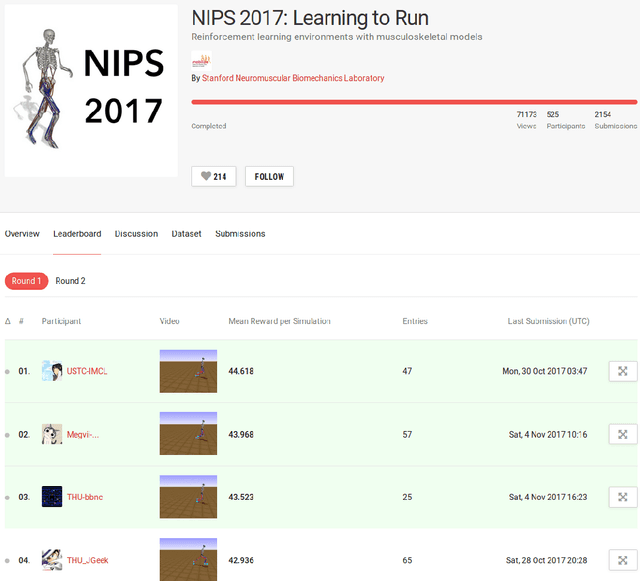
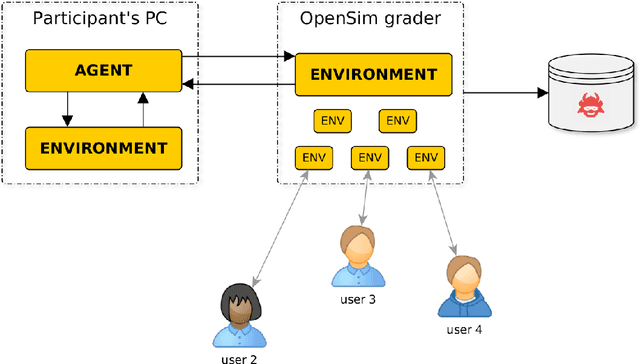
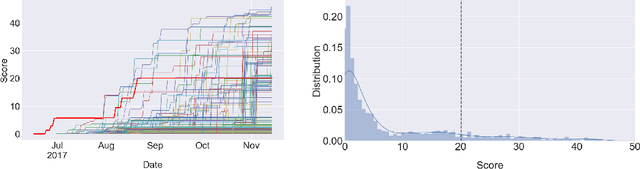
Abstract:Synthesizing physiologically-accurate human movement in a variety of conditions can help practitioners plan surgeries, design experiments, or prototype assistive devices in simulated environments, reducing time and costs and improving treatment outcomes. Because of the large and complex solution spaces of biomechanical models, current methods are constrained to specific movements and models, requiring careful design of a controller and hindering many possible applications. We sought to discover if modern optimization methods efficiently explore these complex spaces. To do this, we posed the problem as a competition in which participants were tasked with developing a controller to enable a physiologically-based human model to navigate a complex obstacle course as quickly as possible, without using any experimental data. They were provided with a human musculoskeletal model and a physics-based simulation environment. In this paper, we discuss the design of the competition, technical difficulties, results, and analysis of the top controllers. The challenge proved that deep reinforcement learning techniques, despite their high computational cost, can be successfully employed as an optimization method for synthesizing physiologically feasible motion in high-dimensional biomechanical systems.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge