Scott A. Small
Detecting Schizophrenia with 3D Structural Brain MRI Using Deep Learning
Jul 07, 2022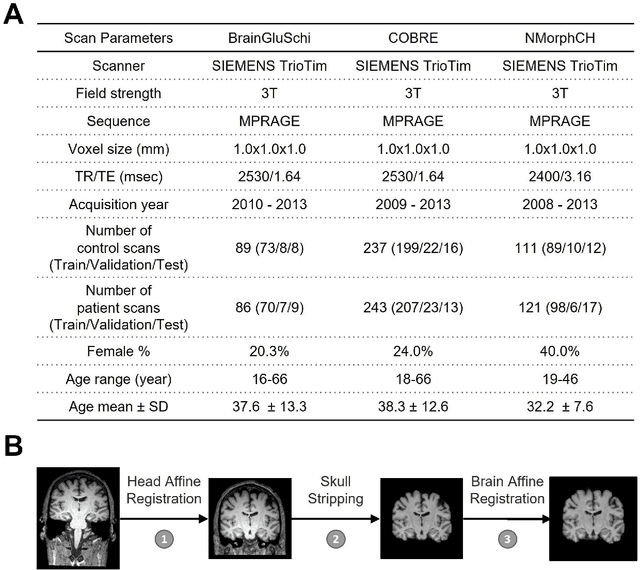
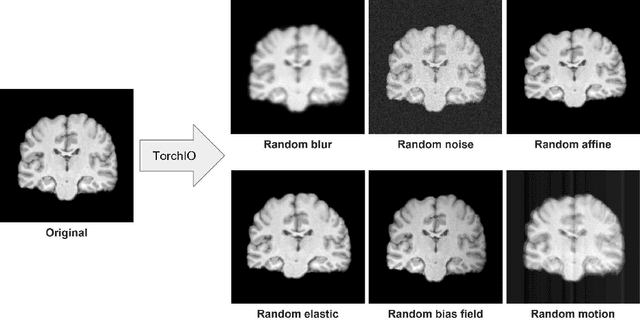
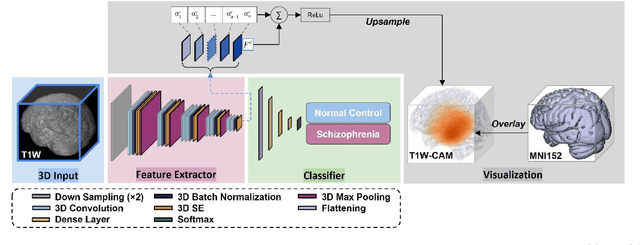
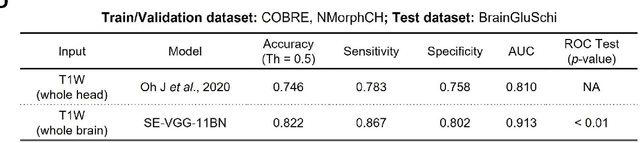
Abstract:Schizophrenia is a chronic neuropsychiatric disorder that causes distinct structural alterations within the brain. We hypothesize that deep learning applied to a structural neuroimaging dataset could detect disease-related alteration and improve classification and diagnostic accuracy. We tested this hypothesis using a single, widely available, and conventional T1-weighted MRI scan, from which we extracted the 3D whole-brain structure using standard post-processing methods. A deep learning model was then developed, optimized, and evaluated on three open datasets with T1-weighted MRI scans of patients with schizophrenia. Our proposed model outperformed the benchmark model, which was also trained with structural MR images using a 3D CNN architecture. Our model is capable of almost perfectly (area under the ROC curve = 0.987) distinguishing schizophrenia patients from healthy controls on unseen structural MRI scans. Regional analysis localized subcortical regions and ventricles as the most predictive brain regions. Subcortical structures serve a pivotal role in cognitive, affective, and social functions in humans, and structural abnormalities of these regions have been associated with schizophrenia. Our finding corroborates that schizophrenia is associated with widespread alterations in subcortical brain structure and the subcortical structural information provides prominent features in diagnostic classification. Together, these results further demonstrate the potential of deep learning to improve schizophrenia diagnosis and identify its structural neuroimaging signatures from a single, standard T1-weighted brain MRI.
Deep Learning Identifies Neuroimaging Signatures of Alzheimer's Disease Using Structural and Synthesized Functional MRI Data
Apr 10, 2021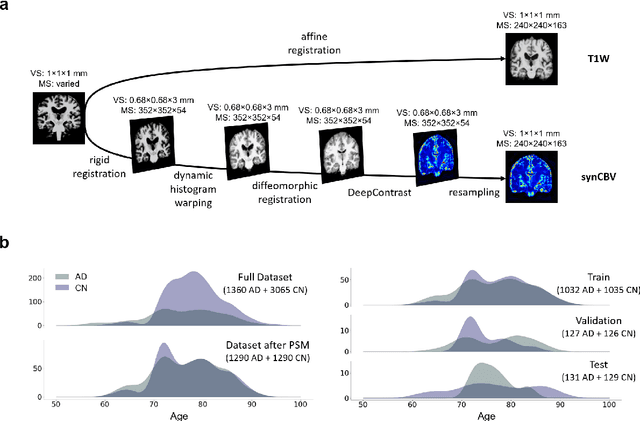
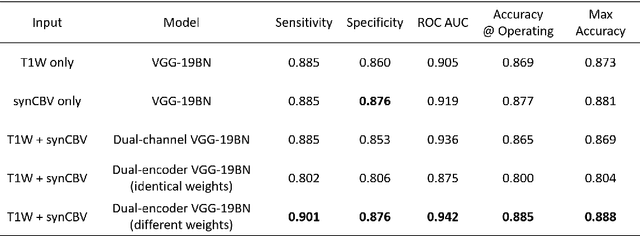
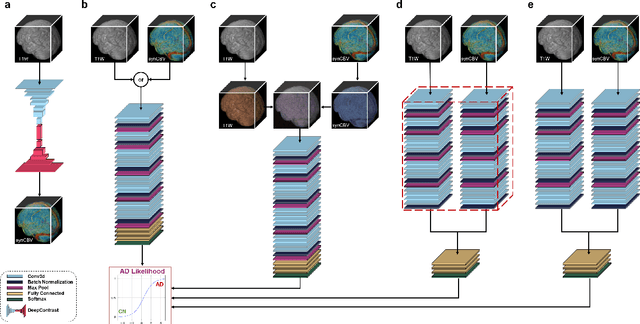
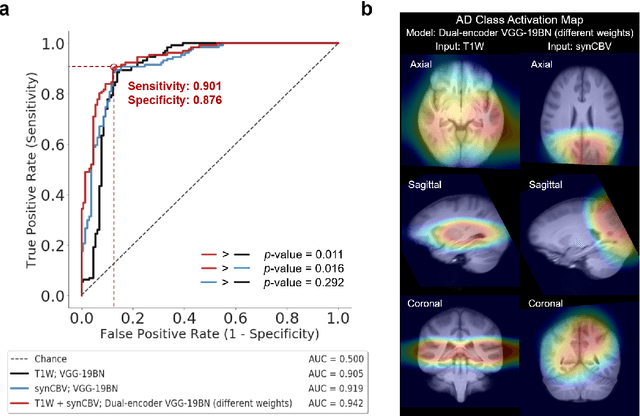
Abstract:Current neuroimaging techniques provide paths to investigate the structure and function of the brain in vivo and have made great advances in understanding Alzheimer's disease (AD). However, the group-level analyses prevalently used for investigation and understanding of the disease are not applicable for diagnosis of individuals. More recently, deep learning, which can efficiently analyze large-scale complex patterns in 3D brain images, has helped pave the way for computer-aided individual diagnosis by providing accurate and automated disease classification. Great progress has been made in classifying AD with deep learning models developed upon increasingly available structural MRI data. The lack of scale-matched functional neuroimaging data prevents such models from being further improved by observing functional changes in pathophysiology. Here we propose a potential solution by first learning a structural-to-functional transformation in brain MRI, and further synthesizing spatially matched functional images from large-scale structural scans. We evaluated our approach by building computational models to discriminate patients with AD from healthy normal subjects and demonstrated a performance boost after combining the structural and synthesized functional brain images into the same model. Furthermore, our regional analyses identified the temporal lobe to be the most predictive structural-region and the parieto-occipital lobe to be the most predictive functional-region of our model, which are both in concordance with previous group-level neuroimaging findings. Together, we demonstrate the potential of deep learning with large-scale structural and synthesized functional MRI to impact AD classification and to identify AD's neuroimaging signatures.
Substituting Gadolinium in Brain MRI Using DeepContrast
Jan 15, 2020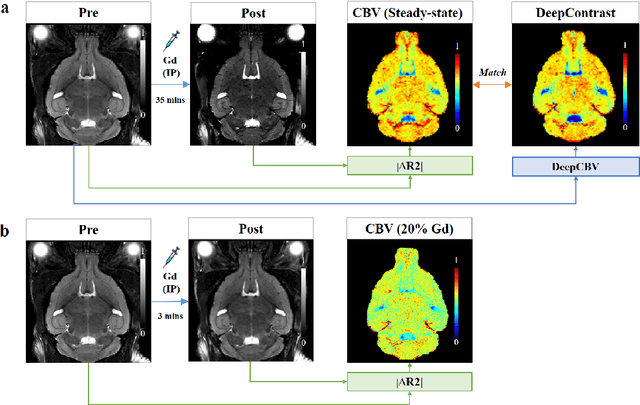
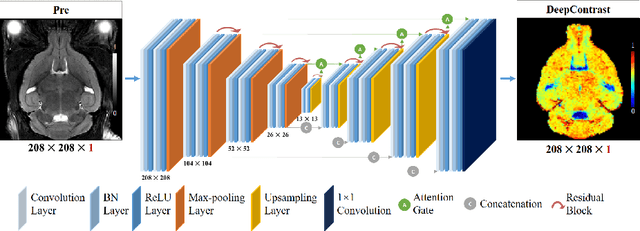
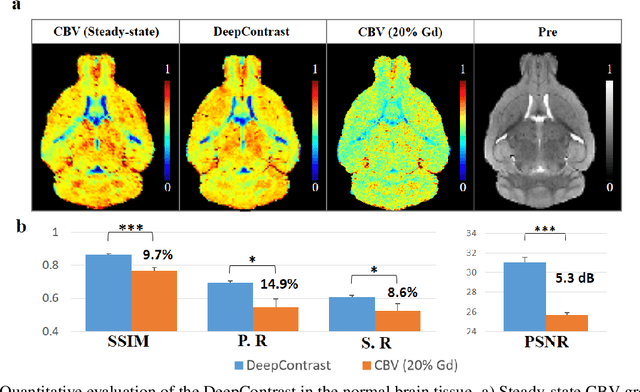
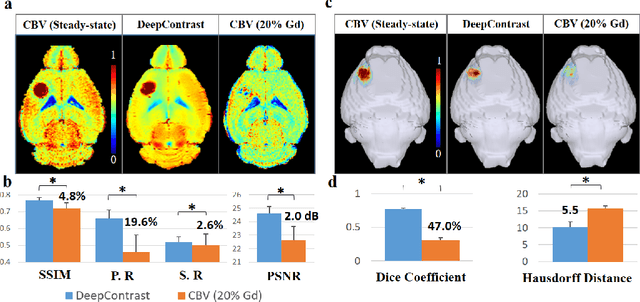
Abstract:Cerebral blood volume (CBV) is a hemodynamic correlate of oxygen metabolism and reflects brain activity and function. High-resolution CBV maps can be generated using the steady-state gadolinium-enhanced MRI technique. Such a technique requires an intravenous injection of exogenous gadolinium based contrast agent (GBCA) and recent studies suggest that the GBCA can accumulate in the brain after frequent use. We hypothesize that endogenous sources of contrast might exist within the most conventional and commonly acquired structural MRI, potentially obviating the need for exogenous contrast. Here, we test this hypothesis by developing and optimizing a deep learning algorithm, which we call DeepContrast, in mice. We find that DeepContrast performs equally well as exogenous GBCA in mapping CBV of the normal brain tissue and enhancing glioblastoma. Together, these studies validate our hypothesis that a deep learning approach can potentially replace the need for GBCAs in brain MRI.
Estimating brain age based on a healthy population with deep learning and structural MRI
Jul 01, 2019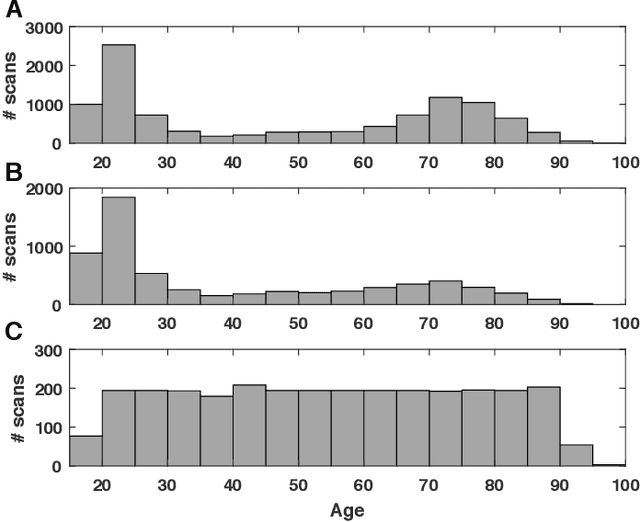
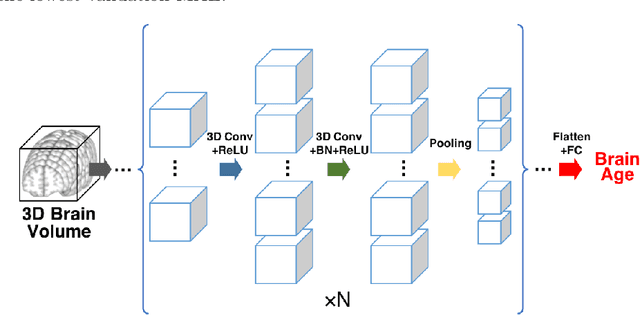
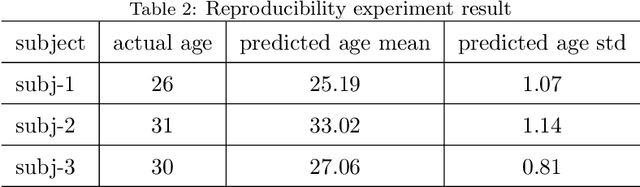
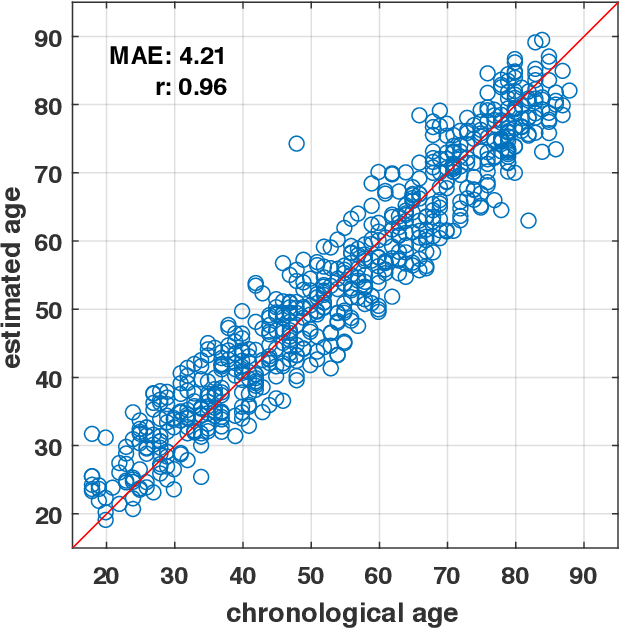
Abstract:Numerous studies have established that estimated brain age, as derived from statistical models trained on healthy populations, constitutes a valuable biomarker that is predictive of cognitive decline and various neurological diseases. In this work, we curate a large-scale heterogeneous dataset (N = 10,158, age range 18 - 97) of structural brain MRIs in a healthy population from multiple publicly-available sources, upon which we train a deep learning model for brain age estimation. The availability of the large-scale dataset enables a more uniform age distribution across adult life-span for effective age estimation with no bias toward certain age groups. We demonstrate that the age estimation accuracy, evaluated with mean absolute error (MAE) and correlation coefficient (r), outperforms previously reported methods in both a hold-out test set reflective of the custom population (MAE = 4.06 years, r = 0.970) and an independent life-span evaluation dataset (MAE = 4.21 years, r = 0.960) on which a previous study has evaluated. We further demonstrate the utility of the estimated age in life-span aging analysis of cognitive functions. Furthermore, we conduct extensive ablation tests and employ feature-attribution techniques to analyze which regions contribute the most predictive value, demonstrating the prominence of the frontal lobe as well as pattern shift across life-span. In summary, we achieve superior age estimation performance confirming the efficacy of deep learning and the added utility of training with data both in larger number and more uniformly distributed than in previous studies. We demonstrate the regional contribution to our brain age predictions through multiple routes and confirm the association of divergence between estimated and chronological brain age with neuropsychological measures.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge