Nanyan Zhu
Deep Learning Identifies Neuroimaging Signatures of Alzheimer's Disease Using Structural and Synthesized Functional MRI Data
Apr 10, 2021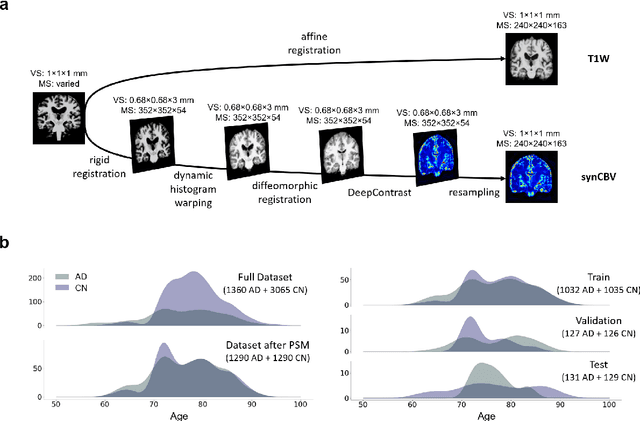
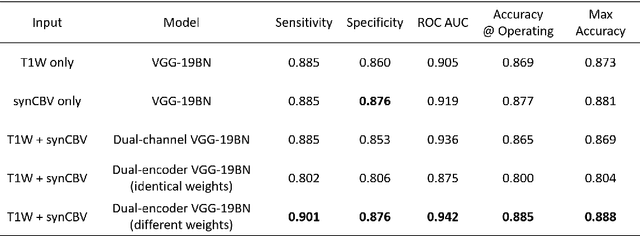
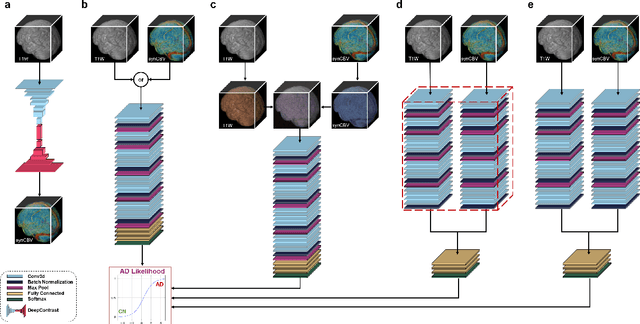
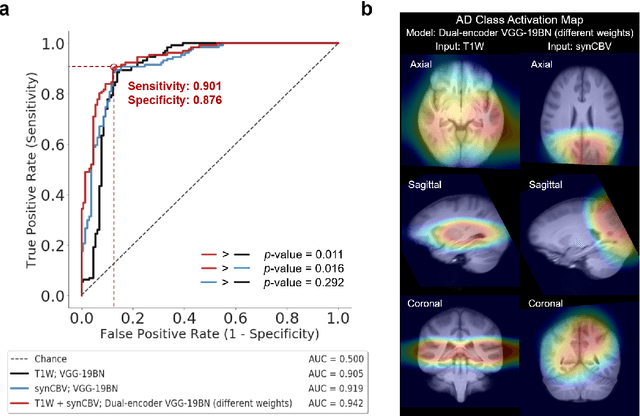
Abstract:Current neuroimaging techniques provide paths to investigate the structure and function of the brain in vivo and have made great advances in understanding Alzheimer's disease (AD). However, the group-level analyses prevalently used for investigation and understanding of the disease are not applicable for diagnosis of individuals. More recently, deep learning, which can efficiently analyze large-scale complex patterns in 3D brain images, has helped pave the way for computer-aided individual diagnosis by providing accurate and automated disease classification. Great progress has been made in classifying AD with deep learning models developed upon increasingly available structural MRI data. The lack of scale-matched functional neuroimaging data prevents such models from being further improved by observing functional changes in pathophysiology. Here we propose a potential solution by first learning a structural-to-functional transformation in brain MRI, and further synthesizing spatially matched functional images from large-scale structural scans. We evaluated our approach by building computational models to discriminate patients with AD from healthy normal subjects and demonstrated a performance boost after combining the structural and synthesized functional brain images into the same model. Furthermore, our regional analyses identified the temporal lobe to be the most predictive structural-region and the parieto-occipital lobe to be the most predictive functional-region of our model, which are both in concordance with previous group-level neuroimaging findings. Together, we demonstrate the potential of deep learning with large-scale structural and synthesized functional MRI to impact AD classification and to identify AD's neuroimaging signatures.
Substituting Gadolinium in Brain MRI Using DeepContrast
Jan 15, 2020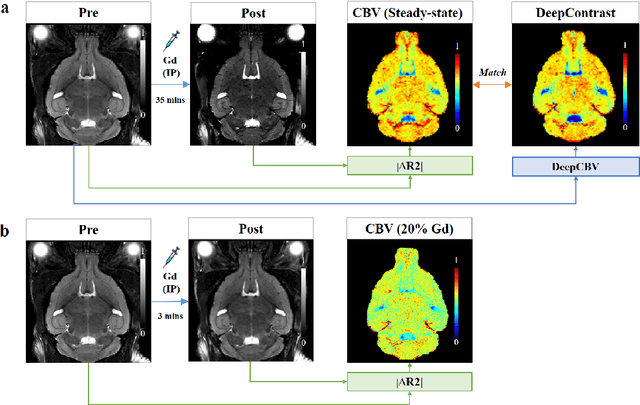
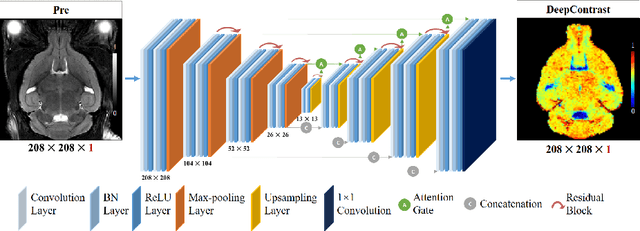
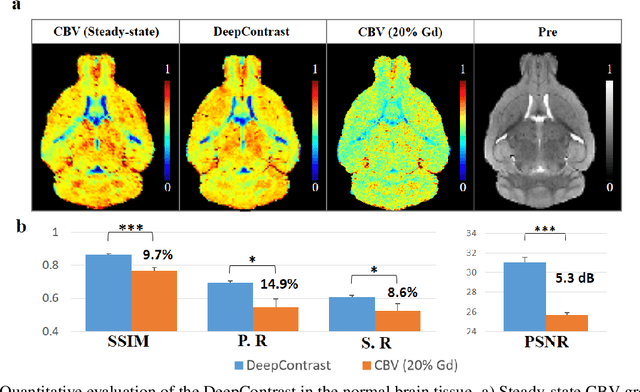
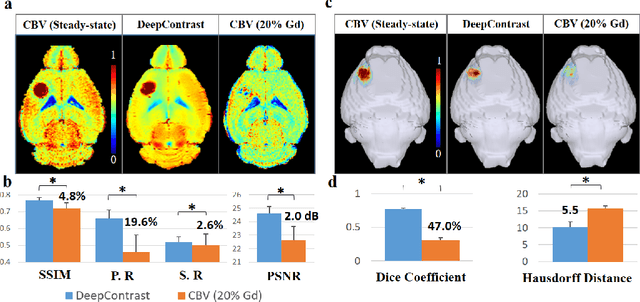
Abstract:Cerebral blood volume (CBV) is a hemodynamic correlate of oxygen metabolism and reflects brain activity and function. High-resolution CBV maps can be generated using the steady-state gadolinium-enhanced MRI technique. Such a technique requires an intravenous injection of exogenous gadolinium based contrast agent (GBCA) and recent studies suggest that the GBCA can accumulate in the brain after frequent use. We hypothesize that endogenous sources of contrast might exist within the most conventional and commonly acquired structural MRI, potentially obviating the need for exogenous contrast. Here, we test this hypothesis by developing and optimizing a deep learning algorithm, which we call DeepContrast, in mice. We find that DeepContrast performs equally well as exogenous GBCA in mapping CBV of the normal brain tissue and enhancing glioblastoma. Together, these studies validate our hypothesis that a deep learning approach can potentially replace the need for GBCAs in brain MRI.
Segmentation with Residual Attention U-Net and an Edge-Enhancement Approach Preserves Cell Shape Features
Jan 15, 2020



Abstract:The ability to extrapolate gene expression dynamics in living single cells requires robust cell segmentation, and one of the challenges is the amorphous or irregularly shaped cell boundaries. To address this issue, we modified the U-Net architecture to segment cells in fluorescence widefield microscopy images and quantitatively evaluated its performance. We also proposed a novel loss function approach that emphasizes the segmentation accuracy on cell boundaries and encourages shape feature preservation. With a 97% sensitivity, 93% specificity, 91% Jaccard similarity, and 95% Dice coefficient, our proposed method called Residual Attention U-Net with edge-enhancement surpassed the state-of-the-art U-Net in segmentation performance as evaluated by the traditional metrics. More remarkably, the same proposed candidate also performed the best in terms of the preservation of valuable shape features, namely area, eccentricity, major axis length, solidity and orientation. These improvements on shape feature preservation can serve as useful assets for downstream cell tracking and quantification of changes in cell statistics or features over time.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge