Qiong Xiao
A Data-Efficient Pan-Tumor Foundation Model for Oncology CT Interpretation
Feb 10, 2025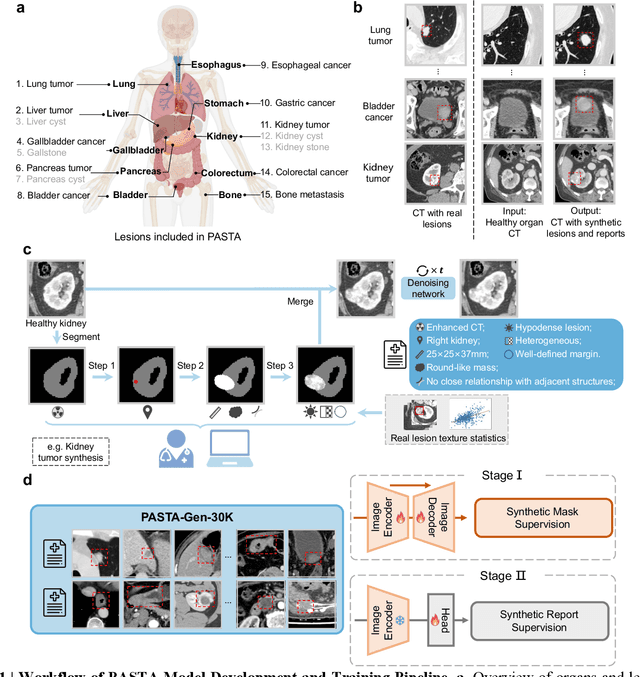
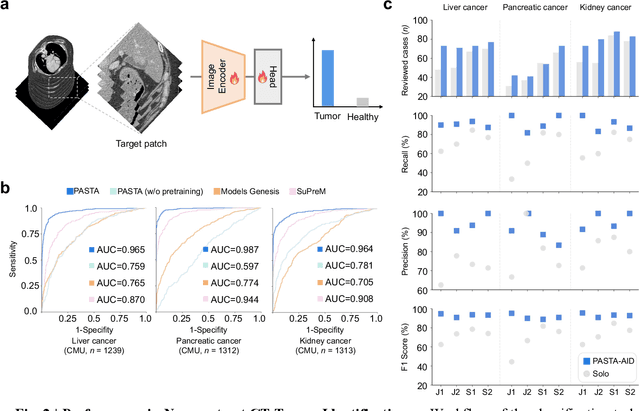
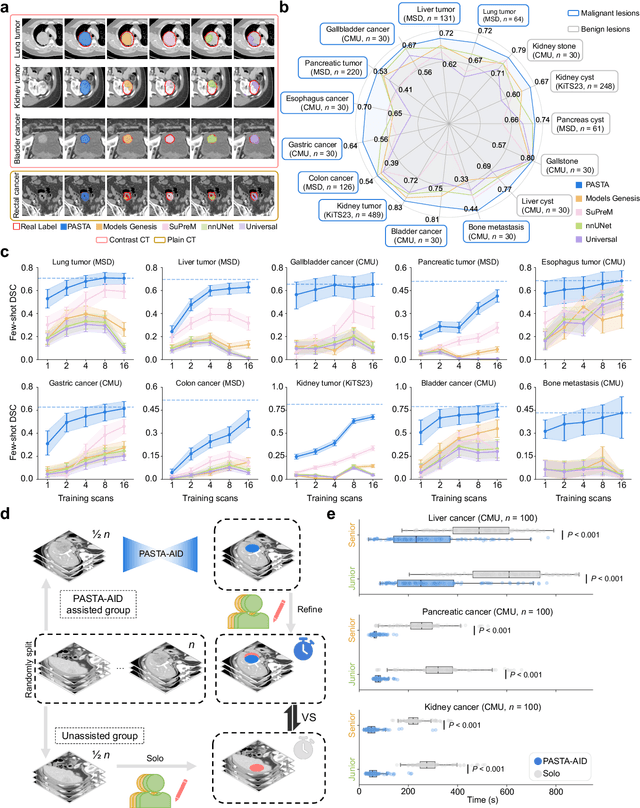
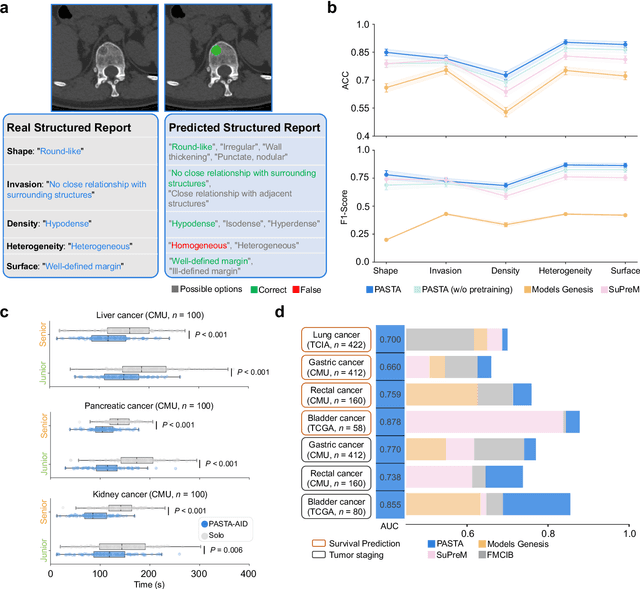
Abstract:Artificial intelligence-assisted imaging analysis has made substantial strides in tumor diagnosis and management. Here we present PASTA, a pan-tumor CT foundation model that achieves state-of-the-art performance on 45 of 46 representative oncology tasks -- including lesion segmentation, tumor detection in plain CT, tumor staging, survival prediction, structured report generation, and cross-modality transfer learning, significantly outperforming the second-best models on 35 tasks. This remarkable advancement is driven by our development of PASTA-Gen, an innovative synthetic tumor generation framework that produces a comprehensive dataset of 30,000 CT scans with pixel-level annotated lesions and paired structured reports, encompassing malignancies across ten organs and five benign lesion types. By leveraging this rich, high-quality synthetic data, we overcome a longstanding bottleneck in the development of CT foundation models -- specifically, the scarcity of publicly available, high-quality annotated datasets due to privacy constraints and the substantial labor required for scaling precise data annotation. Encouragingly, PASTA demonstrates exceptional data efficiency with promising practical value, markedly improving performance on various tasks with only a small amount of real-world data. The open release of both the synthetic dataset and PASTA foundation model effectively addresses the challenge of data scarcity, thereby advancing oncological research and clinical translation.
CT Synthesis with Conditional Diffusion Models for Abdominal Lymph Node Segmentation
Mar 26, 2024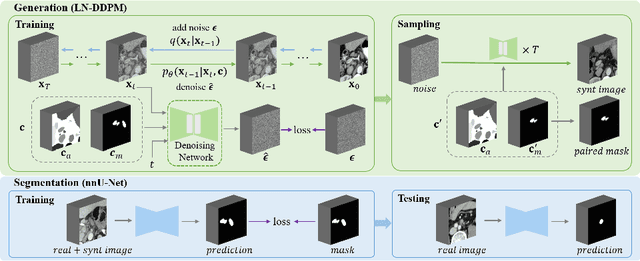
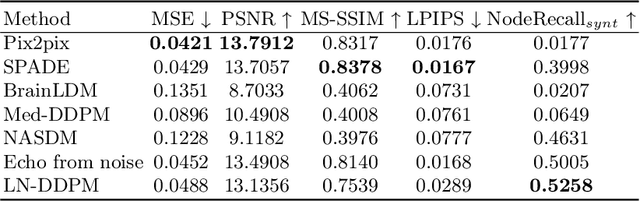
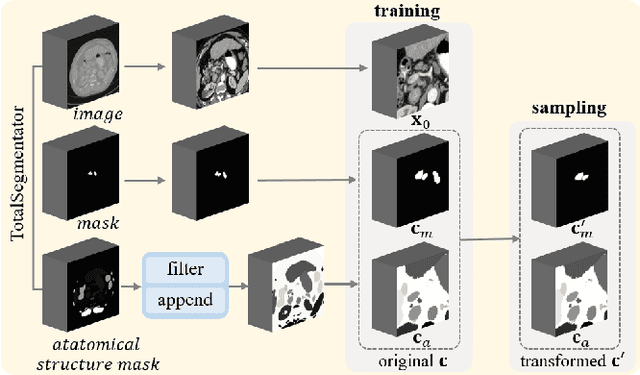
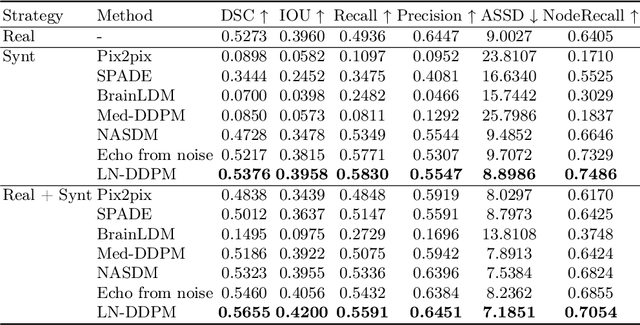
Abstract:Despite the significant success achieved by deep learning methods in medical image segmentation, researchers still struggle in the computer-aided diagnosis of abdominal lymph nodes due to the complex abdominal environment, small and indistinguishable lesions, and limited annotated data. To address these problems, we present a pipeline that integrates the conditional diffusion model for lymph node generation and the nnU-Net model for lymph node segmentation to improve the segmentation performance of abdominal lymph nodes through synthesizing a diversity of realistic abdominal lymph node data. We propose LN-DDPM, a conditional denoising diffusion probabilistic model (DDPM) for lymph node (LN) generation. LN-DDPM utilizes lymph node masks and anatomical structure masks as model conditions. These conditions work in two conditioning mechanisms: global structure conditioning and local detail conditioning, to distinguish between lymph nodes and their surroundings and better capture lymph node characteristics. The obtained paired abdominal lymph node images and masks are used for the downstream segmentation task. Experimental results on the abdominal lymph node datasets demonstrate that LN-DDPM outperforms other generative methods in the abdominal lymph node image synthesis and better assists the downstream abdominal lymph node segmentation task.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge