Mani A Vannan
Neural Multi-Scale Self-Supervised Registration for Echocardiogram Dense Tracking
Jun 18, 2019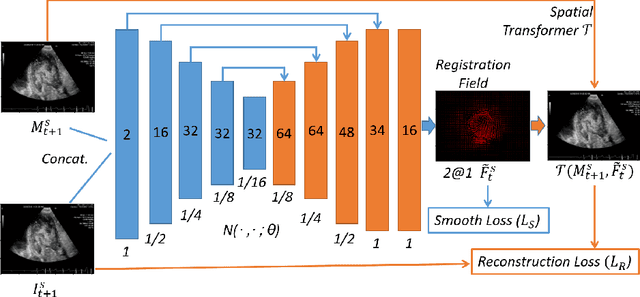
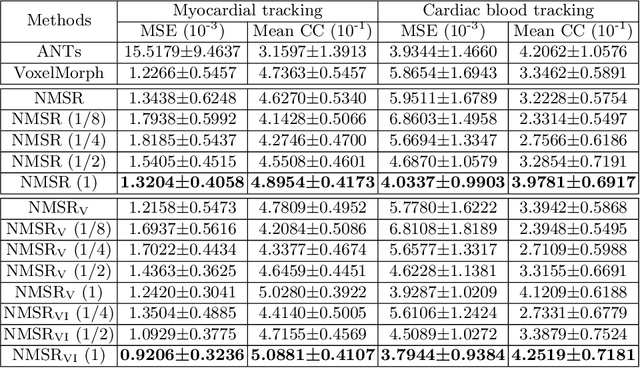
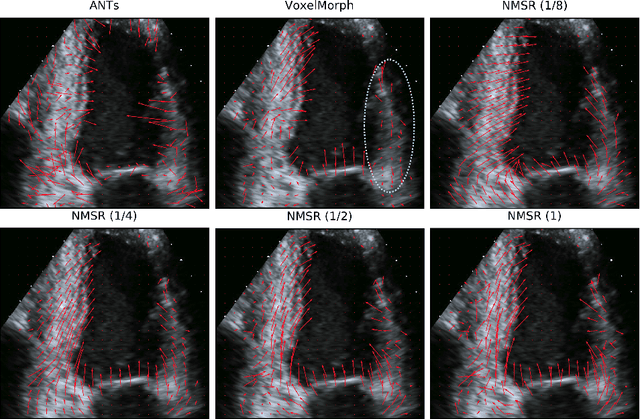
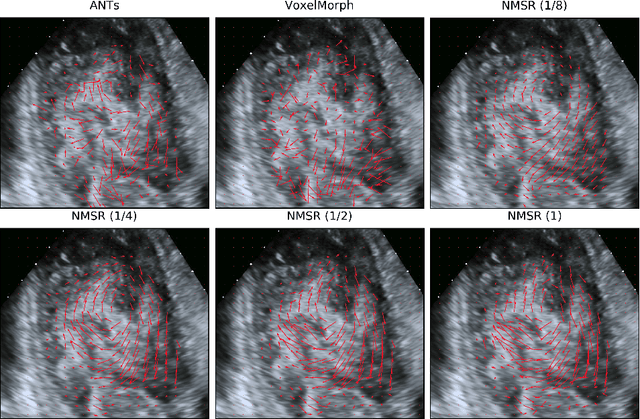
Abstract:Echocardiography has become routinely used in the diagnosis of cardiomyopathy and abnormal cardiac blood flow. However, manually measuring myocardial motion and cardiac blood flow from echocardiogram is time-consuming and error-prone. Computer algorithms that can automatically track and quantify myocardial motion and cardiac blood flow are highly sought after, but have not been very successful due to noise and high variability of echocardiography. In this work, we propose a neural multi-scale self-supervised registration (NMSR) method for automated myocardial and cardiac blood flow dense tracking. NMSR incorporates two novel components: 1) utilizing a deep neural net to parameterize the velocity field between two image frames, and 2) optimizing the parameters of the neural net in a sequential multi-scale fashion to account for large variations within the velocity field. Experiments demonstrate that NMSR yields significantly better registration accuracy than state-of-the-art methods, such as advanced normalization tools (ANTs) and VoxelMorph, for both myocardial and cardiac blood flow dense tracking. Our approach promises to provide a fully automated method for fast and accurate analyses of echocardiograms.
Generative Invertible Networks (GIN): Pathophysiology-Interpretable Feature Mapping and Virtual Patient Generation
Aug 14, 2018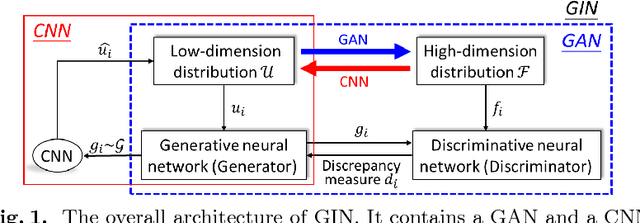
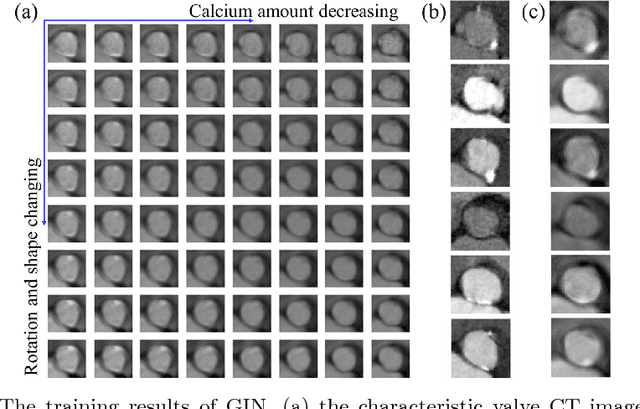
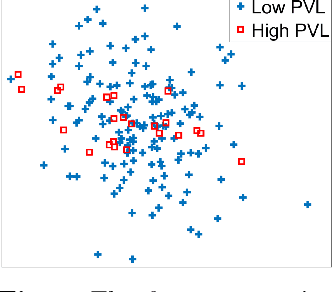
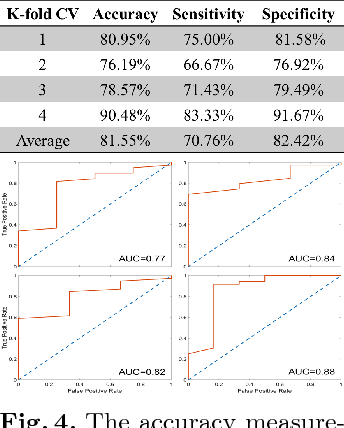
Abstract:Machine learning methods play increasingly important roles in pre-procedural planning for complex surgeries and interventions. Very often, however, researchers find the historical records of emerging surgical techniques, such as the transcatheter aortic valve replacement (TAVR), are highly scarce in quantity. In this paper, we address this challenge by proposing novel generative invertible networks (GIN) to select features and generate high-quality virtual patients that may potentially serve as an additional data source for machine learning. Combining a convolutional neural network (CNN) and generative adversarial networks (GAN), GIN discovers the pathophysiologic meaning of the feature space. Moreover, a test of predicting the surgical outcome directly using the selected features results in a high accuracy of 81.55%, which suggests little pathophysiologic information has been lost while conducting the feature selection. This demonstrates GIN can generate virtual patients not only visually authentic but also pathophysiologically interpretable.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge