Léo Lebrat
NC-Reg : Neural Cortical Maps for Rigid Registration
Jan 26, 2026Abstract:We introduce neural cortical maps, a continuous and compact neural representation for cortical feature maps, as an alternative to traditional discrete structures such as grids and meshes. It can learn from meshes of arbitrary size and provide learnt features at any resolution. Neural cortical maps enable efficient optimization on the sphere and achieve runtimes up to 30 times faster than classic barycentric interpolation (for the same number of iterations). As a proof of concept, we investigate rigid registration of cortical surfaces and propose NC-Reg, a novel iterative algorithm that involves the use of neural cortical feature maps, gradient descent optimization and a simulated annealing strategy. Through ablation studies and subject-to-template experiments, our method demonstrates sub-degree accuracy ($<1^\circ$ from the global optimum), and serves as a promising robust pre-alignment strategy, which is critical in clinical settings.
Multi-View Consistent Wound Segmentation With Neural Fields
Jan 23, 2026Abstract:Wound care is often challenged by the economic and logistical burdens that consistently afflict patients and hospitals worldwide. In recent decades, healthcare professionals have sought support from computer vision and machine learning algorithms. In particular, wound segmentation has gained interest due to its ability to provide professionals with fast, automatic tissue assessment from standard RGB images. Some approaches have extended segmentation to 3D, enabling more complete and precise healing progress tracking. However, inferring multi-view consistent 3D structures from 2D images remains a challenge. In this paper, we evaluate WoundNeRF, a NeRF SDF-based method for estimating robust wound segmentations from automatically generated annotations. We demonstrate the potential of this paradigm in recovering accurate segmentations by comparing it against state-of-the-art Vision Transformer networks and conventional rasterisation-based algorithms. The code will be released to facilitate further development in this promising paradigm.
Wound3DAssist: A Practical Framework for 3D Wound Assessment
Aug 25, 2025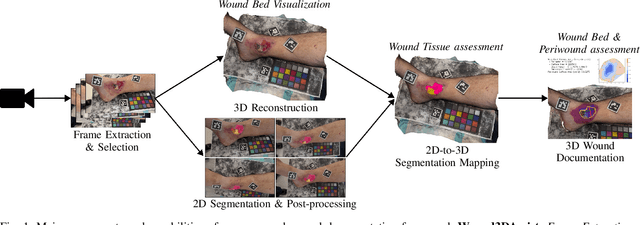
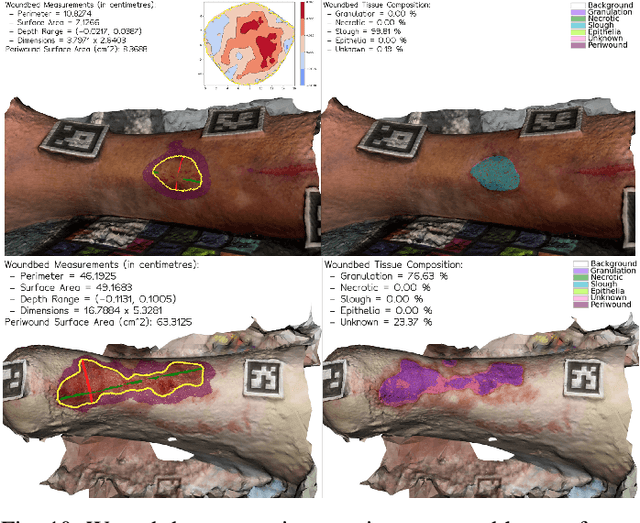
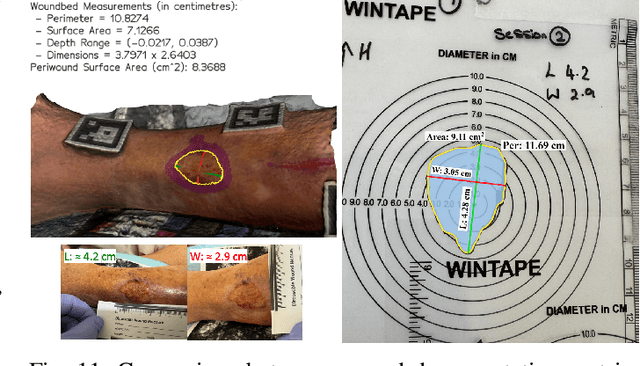
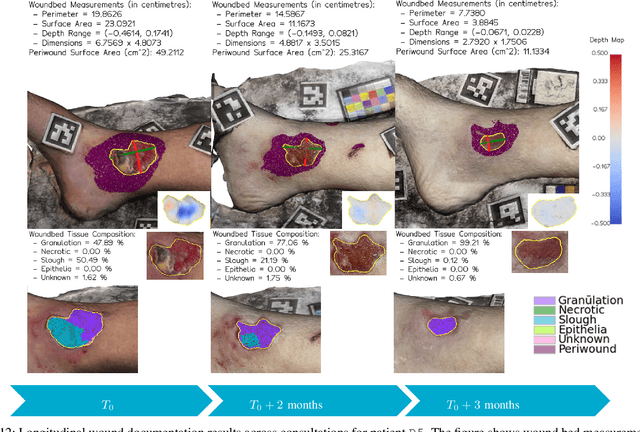
Abstract:Managing chronic wounds remains a major healthcare challenge, with clinical assessment often relying on subjective and time-consuming manual documentation methods. Although 2D digital videometry frameworks aided the measurement process, these approaches struggle with perspective distortion, a limited field of view, and an inability to capture wound depth, especially in anatomically complex or curved regions. To overcome these limitations, we present Wound3DAssist, a practical framework for 3D wound assessment using monocular consumer-grade videos. Our framework generates accurate 3D models from short handheld smartphone video recordings, enabling non-contact, automatic measurements that are view-independent and robust to camera motion. We integrate 3D reconstruction, wound segmentation, tissue classification, and periwound analysis into a modular workflow. We evaluate Wound3DAssist across digital models with known geometry, silicone phantoms, and real patients. Results show that the framework supports high-quality wound bed visualization, millimeter-level accuracy, and reliable tissue composition analysis. Full assessments are completed in under 20 minutes, demonstrating feasibility for real-world clinical use.
Syn3DWound: A Synthetic Dataset for 3D Wound Bed Analysis
Nov 27, 2023


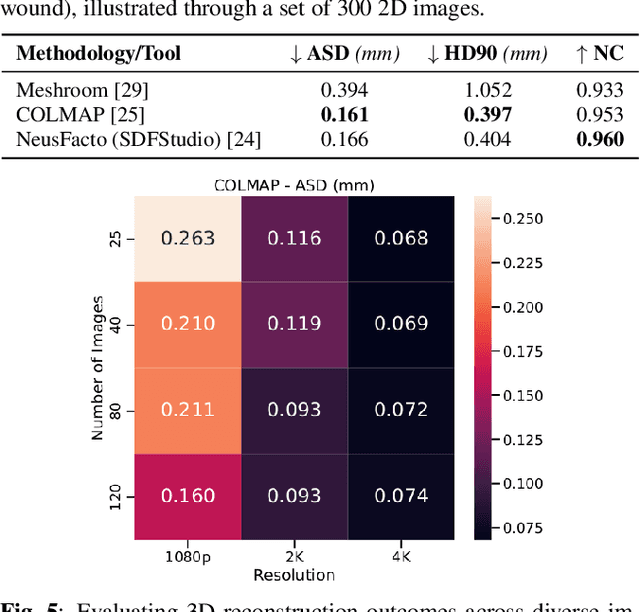
Abstract:Wound management poses a significant challenge, particularly for bedridden patients and the elderly. Accurate diagnostic and healing monitoring can significantly benefit from modern image analysis, providing accurate and precise measurements of wounds. Despite several existing techniques, the shortage of expansive and diverse training datasets remains a significant obstacle to constructing machine learning-based frameworks. This paper introduces Syn3DWound, an open-source dataset of high-fidelity simulated wounds with 2D and 3D annotations. We propose baseline methods and a benchmarking framework for automated 3D morphometry analysis and 2D/3D wound segmentation.
CorticalFlow$^{++}$: Boosting Cortical Surface Reconstruction Accuracy, Regularity, and Interoperability
Jun 14, 2022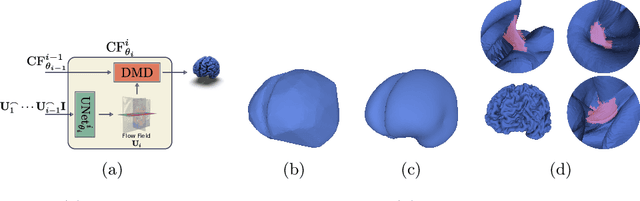
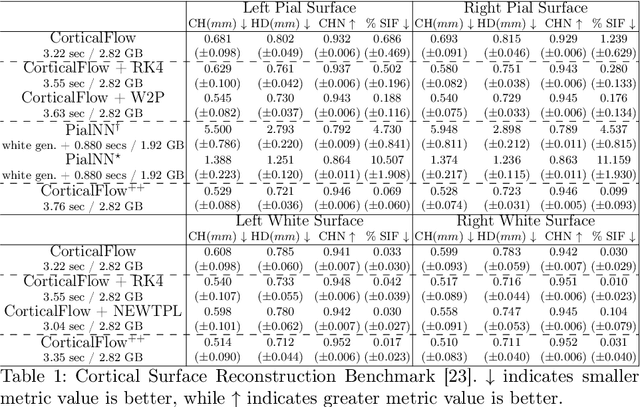
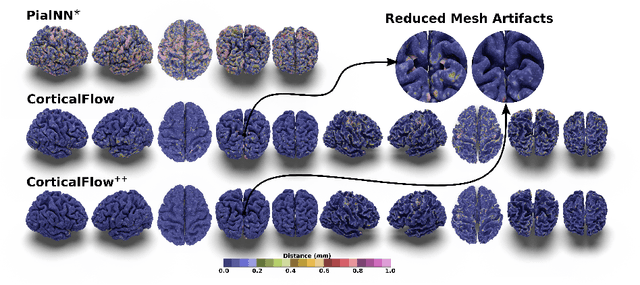
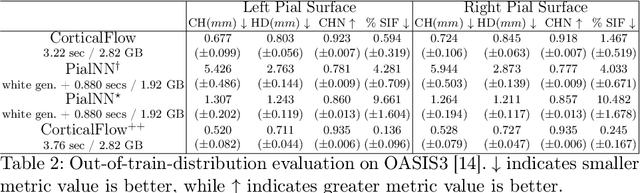
Abstract:The problem of Cortical Surface Reconstruction from magnetic resonance imaging has been traditionally addressed using lengthy pipelines of image processing techniques like FreeSurfer, CAT, or CIVET. These frameworks require very long runtimes deemed unfeasible for real-time applications and unpractical for large-scale studies. Recently, supervised deep learning approaches have been introduced to speed up this task cutting down the reconstruction time from hours to seconds. Using the state-of-the-art CorticalFlow model as a blueprint, this paper proposes three modifications to improve its accuracy and interoperability with existing surface analysis tools, while not sacrificing its fast inference time and low GPU memory consumption. First, we employ a more accurate ODE solver to reduce the diffeomorphic mapping approximation error. Second, we devise a routine to produce smoother template meshes avoiding mesh artifacts caused by sharp edges in CorticalFlow's convex-hull based template. Last, we recast pial surface prediction as the deformation of the predicted white surface leading to a one-to-one mapping between white and pial surface vertices. This mapping is essential to many existing surface analysis tools for cortical morphometry. We name the resulting method CorticalFlow$^{++}$. Using large-scale datasets, we demonstrate the proposed changes provide more geometric accuracy and surface regularity while keeping the reconstruction time and GPU memory requirements almost unchanged.
CorticalFlow: A Diffeomorphic Mesh Deformation Module for Cortical Surface Reconstruction
Jun 06, 2022
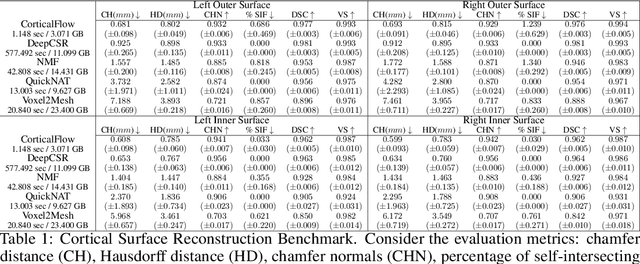
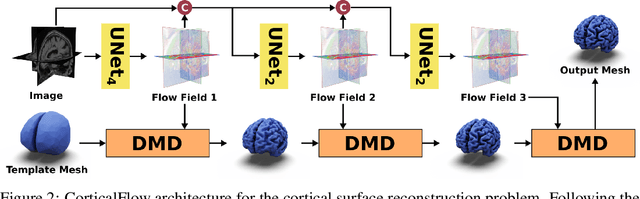
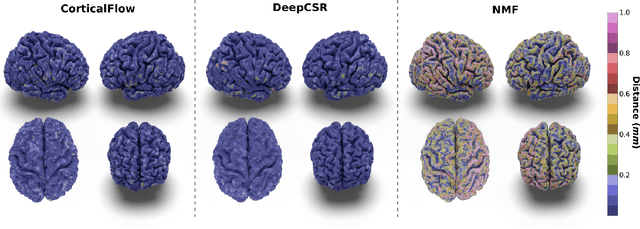
Abstract:In this paper we introduce CorticalFlow, a new geometric deep-learning model that, given a 3-dimensional image, learns to deform a reference template towards a targeted object. To conserve the template mesh's topological properties, we train our model over a set of diffeomorphic transformations. This new implementation of a flow Ordinary Differential Equation (ODE) framework benefits from a small GPU memory footprint, allowing the generation of surfaces with several hundred thousand vertices. To reduce topological errors introduced by its discrete resolution, we derive numeric conditions which improve the manifoldness of the predicted triangle mesh. To exhibit the utility of CorticalFlow, we demonstrate its performance for the challenging task of brain cortical surface reconstruction. In contrast to current state-of-the-art, CorticalFlow produces superior surfaces while reducing the computation time from nine and a half minutes to one second. More significantly, CorticalFlow enforces the generation of anatomically plausible surfaces; the absence of which has been a major impediment restricting the clinical relevance of such surface reconstruction methods.
MongeNet: Efficient Sampler for Geometric Deep Learning
Apr 29, 2021
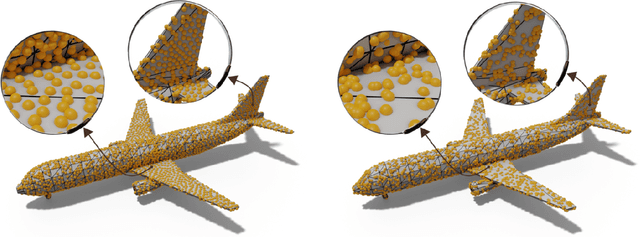


Abstract:Recent advances in geometric deep-learning introduce complex computational challenges for evaluating the distance between meshes. From a mesh model, point clouds are necessary along with a robust distance metric to assess surface quality or as part of the loss function for training models. Current methods often rely on a uniform random mesh discretization, which yields irregular sampling and noisy distance estimation. In this paper we introduce MongeNet, a fast and optimal transport based sampler that allows for an accurate discretization of a mesh with better approximation properties. We compare our method to the ubiquitous random uniform sampling and show that the approximation error is almost half with a very small computational overhead.
Going deeper with brain morphometry using neural networks
Sep 07, 2020
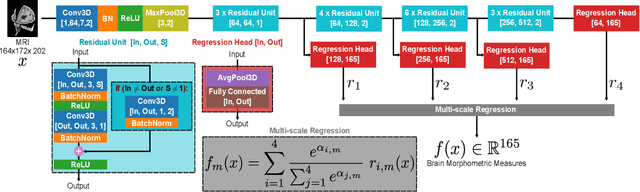
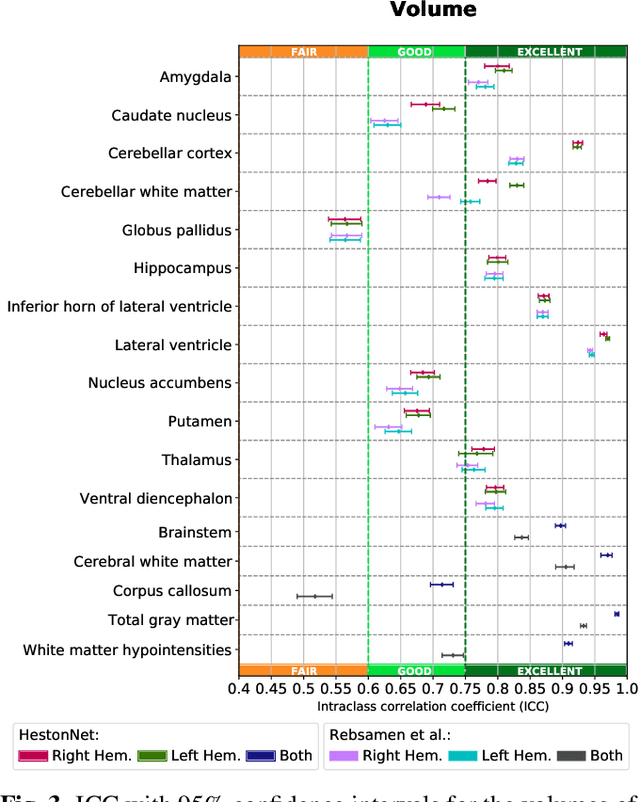
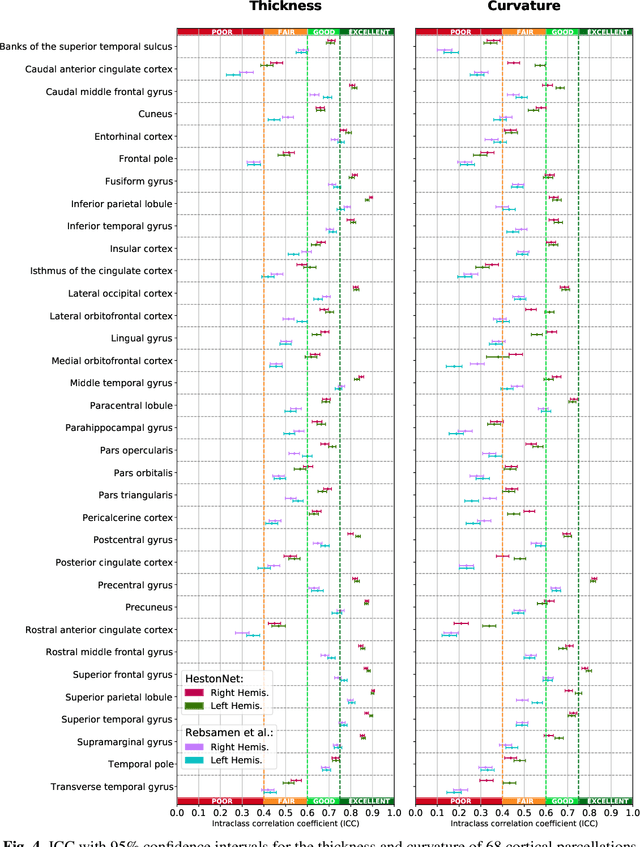
Abstract:Brain morphometry from magnetic resonance imaging (MRI) is a consolidated biomarker for many neurodegenerative diseases. Recent advances in this domain indicate that deep convolutional neural networks can infer morphometric measurements within a few seconds. Nevertheless, the accuracy of the devised model for insightful bio-markers (mean curvature and thickness) remains unsatisfactory. In this paper, we propose a more accurate and efficient neural network model for brain morphometry named HerstonNet. More specifically, we develop a 3D ResNet-based neural network to learn rich features directly from MRI, design a multi-scale regression scheme by predicting morphometric measures at feature maps of different resolutions, and leverage a robust optimization method to avoid poor quality minima and reduce the prediction variance. As a result, HerstonNet improves the existing approach by 24.30% in terms of intraclass correlation coefficient (agreement measure) to FreeSurfer silver-standards while maintaining a competitive run-time.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge