Krishna Agarwal
Reliable Policy Iteration: Performance Robustness Across Architecture and Environment Perturbations
Dec 12, 2025Abstract:In a recent work, we proposed Reliable Policy Iteration (RPI), that restores policy iteration's monotonicity-of-value-estimates property to the function approximation setting. Here, we assess the robustness of RPI's empirical performance on two classical control tasks -- CartPole and Inverted Pendulum -- under changes to neural network and environmental parameters. Relative to DQN, Double DQN, DDPG, TD3, and PPO, RPI reaches near-optimal performance early and sustains this policy as training proceeds. Because deep RL methods are often hampered by sample inefficiency, training instability, and hyperparameter sensitivity, our results highlight RPI's promise as a more reliable alternative.
packetLSTM: Dynamic LSTM Framework for Streaming Data with Varying Feature Space
Oct 22, 2024



Abstract:We study the online learning problem characterized by the varying input feature space of streaming data. Although LSTMs have been employed to effectively capture the temporal nature of streaming data, they cannot handle the dimension-varying streams in an online learning setting. Therefore, we propose a dynamic LSTM-based novel method, called packetLSTM, to model the dimension-varying streams. The packetLSTM's dynamic framework consists of an evolving packet of LSTMs, each dedicated to processing one input feature. Each LSTM retains the local information of its corresponding feature, while a shared common memory consolidates global information. This configuration facilitates continuous learning and mitigates the issue of forgetting, even when certain features are absent for extended time periods. The idea of utilizing one LSTM per feature coupled with a dimension-invariant operator for information aggregation enhances the dynamic nature of packetLSTM. This dynamic nature is evidenced by the model's ability to activate, deactivate, and add new LSTMs as required, thus seamlessly accommodating varying input dimensions. The packetLSTM achieves state-of-the-art results on five datasets, and its underlying principle is extended to other RNN types, like GRU and vanilla RNN.
Online Learning under Haphazard Input Conditions: A Comprehensive Review and Analysis
Apr 07, 2024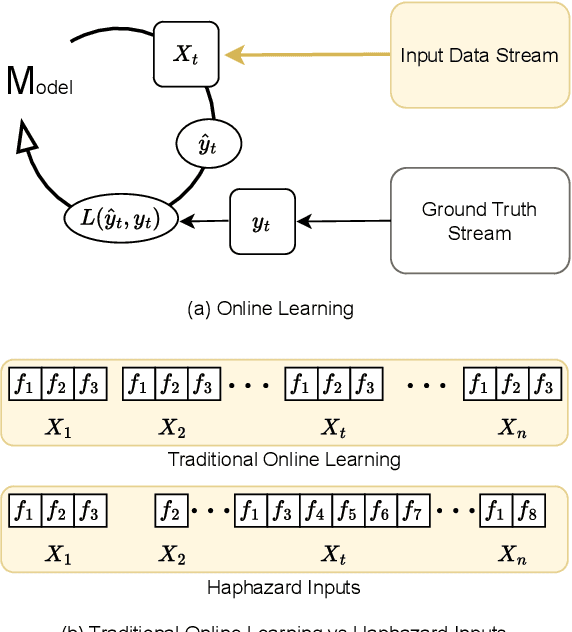
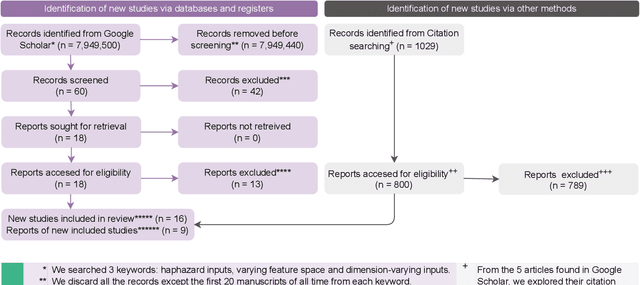
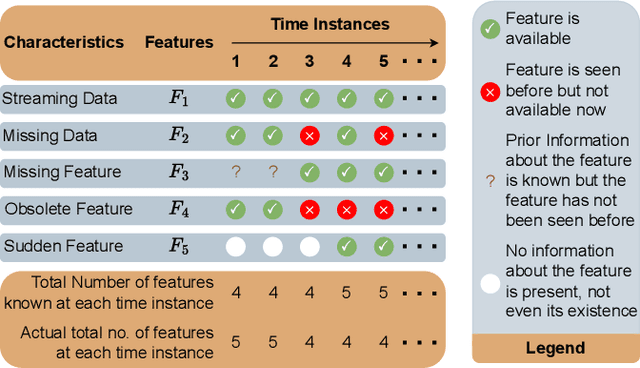
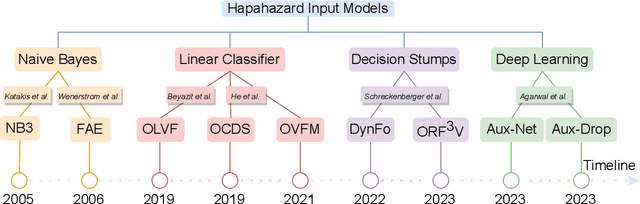
Abstract:The domain of online learning has experienced multifaceted expansion owing to its prevalence in real-life applications. Nonetheless, this progression operates under the assumption that the input feature space of the streaming data remains constant. In this survey paper, we address the topic of online learning in the context of haphazard inputs, explicitly foregoing such an assumption. We discuss, classify, evaluate, and compare the methodologies that are adept at modeling haphazard inputs, additionally providing the corresponding code implementations and their carbon footprint. Moreover, we classify the datasets related to the field of haphazard inputs and introduce evaluation metrics specifically designed for datasets exhibiting imbalance. The code of each methodology can be found at https://github.com/Rohit102497/HaphazardInputsReview
MiShape: 3D Shape Modelling of Mitochondria in Microscopy
Mar 02, 2023



Abstract:Fluorescence microscopy is a quintessential tool for observing cells and understanding the underlying mechanisms of life-sustaining processes of all living organisms. The problem of extracting 3D shape of mitochondria from fluorescence microscopy images remains unsolved due to the complex and varied shapes expressed by mitochondria and the poor resolving capacity of these microscopes. We propose an approach to bridge this gap by learning a shape prior for mitochondria termed as MiShape, by leveraging high-resolution electron microscopy data. MiShape is a generative model learned using implicit representations of mitochondrial shapes. It provides a shape distribution that can be used to generate infinite realistic mitochondrial shapes. We demonstrate the representation power of MiShape and its utility for 3D shape reconstruction given a single 2D fluorescence image or a small 3D stack of 2D slices. We also showcase applications of our method by deriving simulated fluorescence microscope datasets that have realistic 3D ground truths for the problem of 2D segmentation and microscope-to-microscope transformation.
Single-shot multispectral quantitative phase imaging using deep neural network
Jan 05, 2022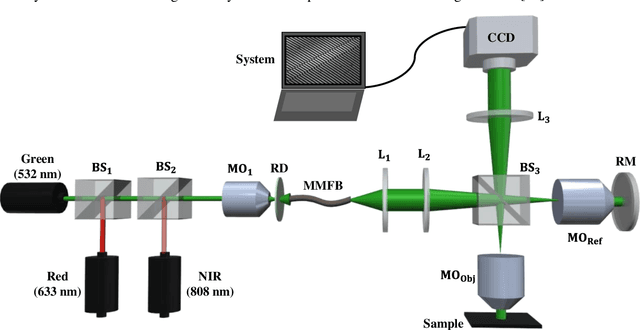
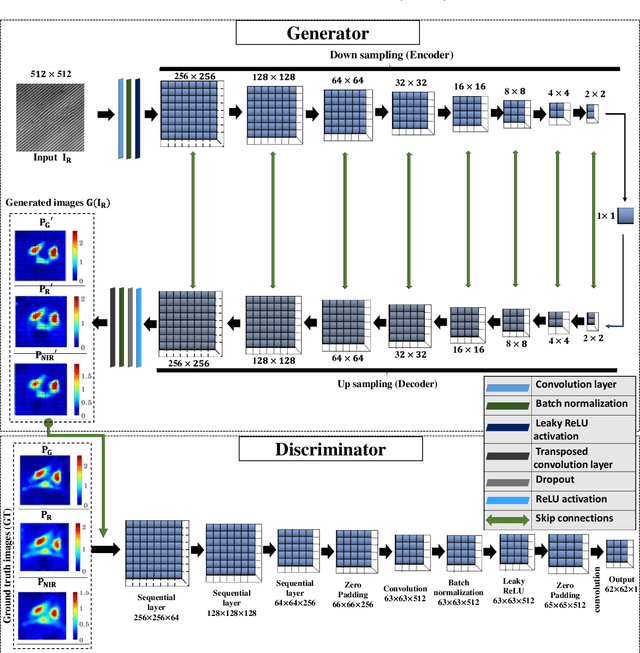
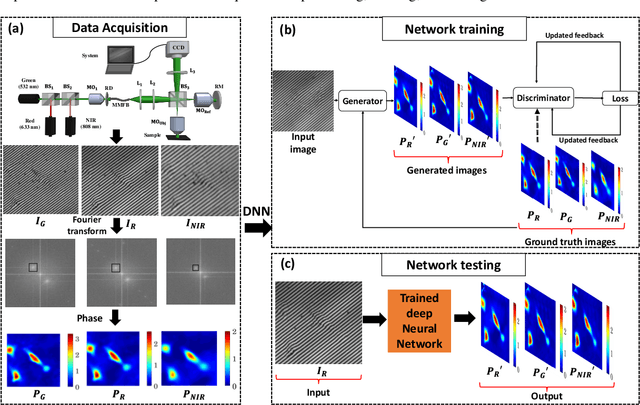

Abstract:Multi-spectral quantitative phase imaging (MS-QPI) is a cutting-edge label-free technique to determine the morphological changes, refractive index variations and spectroscopic information of the specimens. The bottleneck to implement this technique to extract quantitative information, is the need of more than two measurements for generating MS-QPI images. We propose a single-shot MS-QPI technique using highly spatially sensitive digital holographic microscope assisted with deep neural network (DNN). Our method first acquires the interferometric datasets corresponding to multiple wavelengths ({\lambda}=532, 633 and 808 nm used here). The acquired datasets are used to train generative adversarial network (GAN) to generate multi-spectral quantitative phase maps from a single input interferogram. The network is trained and validated on two different samples, the optical waveguide and a MG63 osteosarcoma cells. Further, validation of the framework is performed by comparing the predicted phase maps with experimentally acquired and processed multi-spectral phase maps. The current MS-QPI+DNN framework can further empower spectroscopic QPI to improve the chemical specificity without complex instrumentation and color-cross talk.
Physics-guided Loss Functions Improve Deep Learning Performance in Inverse Scattering
Nov 13, 2021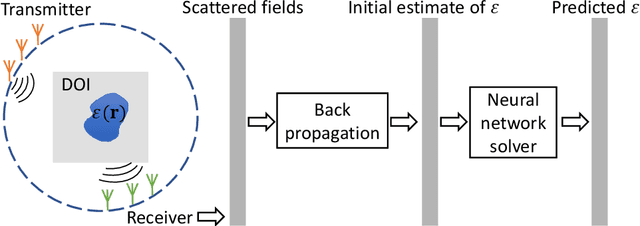
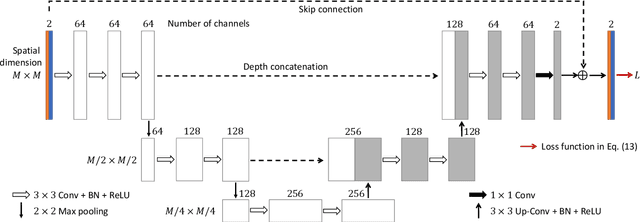
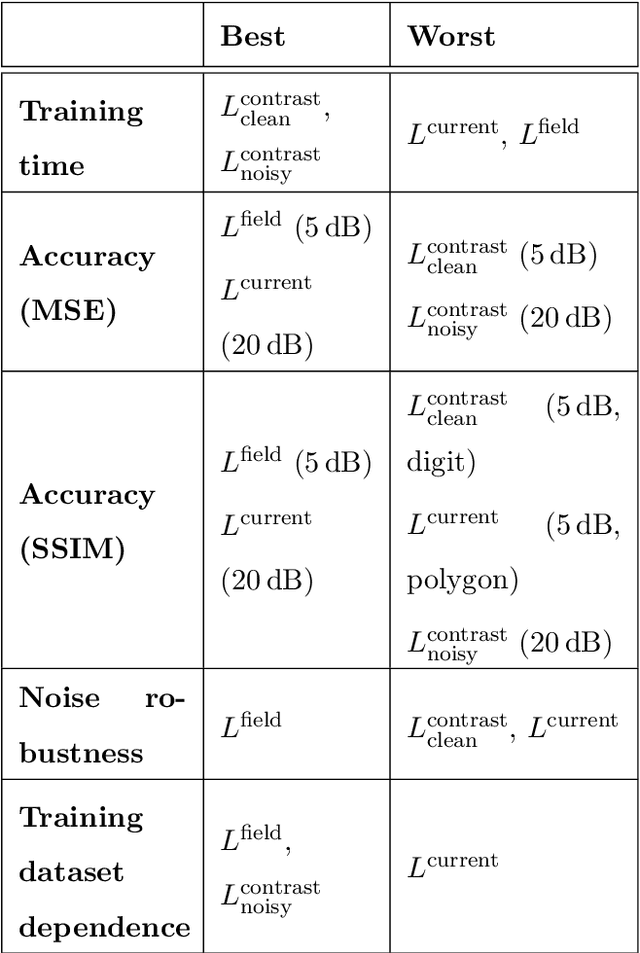

Abstract:Solving electromagnetic inverse scattering problems (ISPs) is challenging due to the intrinsic nonlinearity, ill-posedness, and expensive computational cost. Recently, deep neural network (DNN) techniques have been successfully applied on ISPs and shown potential of superior imaging over conventional methods. In this paper, we analyse the analogy between DNN solvers and traditional iterative algorithms and discuss how important physical phenomena cannot be effectively incorporated in the training process. We show the importance of including near-field priors in the learning process of DNNs. To this end, we propose new designs of loss functions which incorporate multiple-scattering based near-field quantities (such as scattered fields or induced currents within domain of interest). Effects of physics-guided loss functions are studied using a variety of numerical experiments. Pros and cons of the investigated ISP solvers with different loss functions are summarized.
Auxiliary Network: Scalable and agile online learning for dynamic system with inconsistently available inputs
Aug 26, 2020


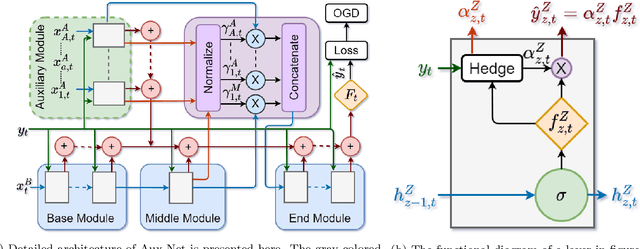
Abstract:Streaming classification methods assume the number of input features is fixed and always received. But in many real-world scenarios demand is some input features are reliable while others are unreliable or inconsistent. In this paper, we propose a novel deep learning-based model called Auxiliary Network (Aux-Net), which is scalable and agile. It employs a weighted ensemble of classifiers to give a final outcome. The Aux-Net model is based on the hedging algorithm and online gradient descent. It employs a model of varying depth in an online setting using single pass learning. Aux-Net is a foundational work towards scalable neural network model for a dynamic complex environment requiring ad hoc or inconsistent input data. The efficacy of Aux-Net is shown on public dataset.
Simulation-supervised deep learning for analysing organelles states and behaviour in living cells
Aug 26, 2020
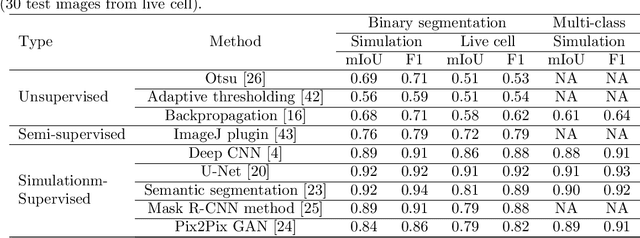

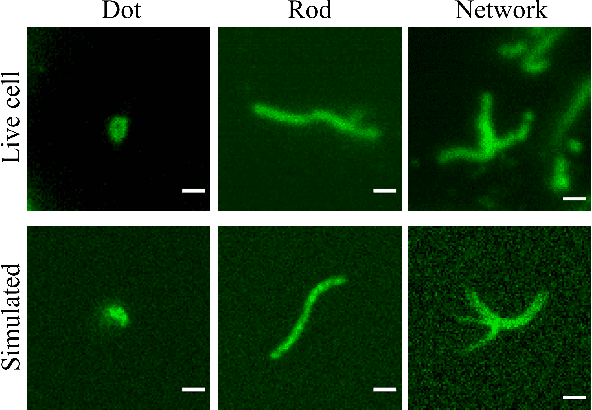
Abstract:In many real-world scientific problems, generating ground truth (GT) for supervised learning is almost impossible. The causes include limitations imposed by scientific instrument, physical phenomenon itself, or the complexity of modeling. Performing artificial intelligence (AI) tasks such as segmentation, tracking, and analytics of small sub-cellular structures such as mitochondria in microscopy videos of living cells is a prime example. The 3D blurring function of microscope, digital resolution from pixel size, optical resolution due to the character of light, noise characteristics, and complex 3D deformable shapes of mitochondria, all contribute to making this problem GT hard. Manual segmentation of 100s of mitochondria across 1000s of frames and then across many such videos is not only herculean but also physically inaccurate because of the instrument and phenomena imposed limitations. Unsupervised learning produces less than optimal results and accuracy is important if inferences relevant to therapy are to be derived. In order to solve this unsurmountable problem, we bring modeling and deep learning to a nexus. We show that accurate physics based modeling of microscopy data including all its limitations can be the solution for generating simulated training datasets for supervised learning. We show here that our simulation-supervised segmentation approach is a great enabler for studying mitochondrial states and behaviour in heart muscle cells, where mitochondria have a significant role to play in the health of the cells. We report unprecedented mean IoU score of 91% for binary segmentation (19% better than the best performing unsupervised approach) of mitochondria in actual microscopy videos of living cells. We further demonstrate the possibility of performing multi-class classification, tracking, and morphology associated analytics at the scale of individual mitochondrion.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge